Abstract
Aims: Early conduction abnormalities and need for pacemaking after transcatheter aortic valve implantation (TAVI) is well recognised. It is still unknown, however, if these conduction abnormalities are persistent, and what is the need for permanent pacemaking after 1-month follow-up. In this prospective study, we examined the incidence of post-procedural and 6-month conduction abnormalities and need for permanent pacemaking after TAVI.
Methods and results: We examined the 12-lead electrocardiogram (ECG) of 91 consecutive patients in whom a Medtronic CoreValve ReValving System was implanted between November 2005 and April 2009. We evaluated the ECGs before treatment, after treatment, at 1-month and 6-month follow-up. The requirement and timing of permanent pacemaking was documented. The mean age of patients was 81±7 years and the mean logistic EuroSCORE was 16±9%. Median duration of follow-up was 213 days (IQR 64, 519). There was a 39% increase in the frequency of LBBB after TAVI (15% before treatment vs. 54% after treatment, p<0.001). Importantly, there was no significant change in the frequency of LBBB from after treatment to 1- or 6-month follow-up (54% after treatment vs. 42% at 1-month follow-up, p=0.45, and 54% after treatment vs. 45% at 6-month follow-up, p=0.39). Permanent pacemaking was required in 17/91 (19%) of patients. A permanent pacemaker was implanted in 8/17 patients (47%) within seven days of TAVI, in 6/17 (35%) at 7-30 days, and in 3/17 (18%) after 30 days. Male gender, previous myocardial infarction, pre-existing right bundle branch block, actual diameter (mm) of the inflow portion of the CoreValve frame post-implantation and depth of implantation were predictors for new LBBB; pre-treatment QRS duration (msec) and septal wall thickness were predictors for permanent pacemaking.
Conclusions: These results suggest that early conduction abnormalities occurring after TAVI persist at 6-months follow-up. Patient-related, anatomical-related, and procedure-related factors need to be considered in the pathogenesis of conduction abnormalities after TAVI.
Introduction
Early conduction abnormalities and need for pacemaking is well recognised after transcatheter aortic valve implantation (TAVI)1-4. The occurrence of conduction abnormalities is not surprising when one considers the anatomical proximity of the aortic valvar complex to the conduction system5. Furthermore, pre-existing conduction abnormalities are common in patients with aortic valvar stenosis6,7.
In our previous work, we demonstrated a 40% increase in the occurrence of LBBB after TAVI1. Although there was a non-significant decrease in the frequency of LBBB from the time after implantation to 1-month follow-up, there was a significant decrease in QRS duration suggesting an improvement in intraventricular conduction. Furthermore, a correlation was found between the depth of implantation of the prosthesis within the left ventricular outflow tract and the occurrence left bundle branch block. Although the clinical implications of new LBBB acquired after TAVI are yet unknown, it is apparent from the surgical literature that new and persistent bundle branch block acquired after aortic valve replacement is associated with an increased risk of subsequent arrhythmic events, such as syncope, AV dissociation, and sudden death8. Furthermore, left bundle branch block can be associated with left ventricular mechanical dyssynchrony, left ventricular remodelling and impaired systolic and diastolic heart function9.
It is still unknown, however, if conduction abnormalities occurring after TAVI are persistent, and what is the need for permanent pacemaking after 1-month follow-up. In this prospective study, we examined the incidence of conduction abnormalities up to 6-month follow-up and need for permanent pacemaking. Furthermore, we sought to identify predictors of left bundle branch block and need for permanent pacemaking acquired after TAVI.
Methods
Patients
We prospectively enrolled consecutive patients with severe aortic stenosis in whom a CoreValve ReValving System (Medtronic, Minneapolis, MN, USA) was implanted between November 2005 and April 2009. For a patient to undergo TAVI, a Heart Team (specifically an interventional cardiologist and cardiac surgeon) had to agree that surgical aortic valve replacement would be associated with either high or prohibitive risk.
Inclusion and exclusion criteria for implantation of the CoreValve ReValving System are in accordance with the 18 Fr Expanded Evaluation Registry study criteria and have been described elsewhere10.
Description of the device and procedure
The CoreValve bioprosthesis consists of a self-expanding nitinol tri-level frame to which is sewn a trileaflet porcine pericardial heart valve. The non-cylindrical frame design exhibits three different diameters associated with three different degrees of radial stiffness. In particular, the (lower) inflow level of the frame exerts high radial stiffness for secure intra-annular anchoring. This inflow portion of the frame has been implicated in the development of conduction abnormalities depending on its depth of implantation1.
The prosthesis is currently available in two sizes based on the diameter of its inflow portion (or ventricular end): 26 or 29 mm. Selection of the prosthesis size is dependent on measurements of the aortic root and ascending aorta5.
Technical details of the procedure have been previously published11. Briefly, a temporary pacemaker was implanted via the femoral vein and positioned in the right ventricle at the beginning of the procedure. Balloon aortic valvuloplasty was performed in all patients before valve implantation. Positioning and deployment of the device was guided by fluoroscopic and angiographic imaging. During our initial experience, it was recommended to position the ventricular edge of the prosthesis 10-12 mm below the lower edge of the non-coronary leaflet. As a result of our previous work relating depth of implantation to the development of conduction abnormalities, we now intend to position the ventricular edge of the prosthesis approximately 6-7 mm below the lower edge of the non-coronary leaflet1.
Following valve implantation, the temporary pacemaker was left in place for 24-72 hours and subsequently removed in the absence of high-degree atrioventricular block. Patients were further monitored by telemetry until discharge.
Collection of ECG and pacemaking data
Twelve-lead electrocardiographic recordings were obtained before treatment, after treatment, at 1-month and 6-month follow-up and examined by a core laboratory (Cardialysis, Rotterdam, The Netherlands). More specifically, the ECGs were analysed for rhythm, heart rate (beats/min), PR, QRS, and corrected QT intervals (all in milliseconds), and the presence of atrioventricular block (first, second, and third degree).
In addition, the diagnostic criteria endorsed by the World Health Organisation and International Society and Federation for Cardiology Task Force were used to code for right and left fascicular hemi block and right and left bundle branch block12.
A continuous 3-lead rhythm strip was continuously recorded and stored electronically. We subsequently analysed the rhythm strips for new widening of the QRS duration (i.e., ≥120 msec) and further categorised the event as occurring either before or after valve implantation.
We documented the need for temporary or permanent pacemaking. Furthermore, the indication and timing of permanent pacemaker implantation from valve implantation (days) was recorded.
Quantitative angiographic measurements (depth of implantation of the frame, percent expansion of the inflow portion of the frame, aortic root angle)
Quantitative angiographic analysis was performed using CAAS 5.4 software (Pie Medical, Maastricht, The Netherlands). Calibration was achieved using a graduated pigtail with radiopaque markers separated 10 mm apart.
We measured the depth of implantation of the frame defined as the distance from the lower edge of the non-coronary and left coronary leaflet to the ventricular edge of the frame. We sought to correlate the depth of implantation of the frame to the occurrence of left bundle branch block acquired specifically after valve implantation (as opposed to new-onset left bundle branch block occurring before valve implantation) (Figure 1).
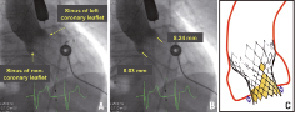
Figure 1. Measurement of depth of implantation from the base of the non-coronary and left coronary sinus to the ventricular end of the bioprosthesis. (A) Contrast aortography performed after CoreValve implantation. (B) Same figure as in A but includes measurements of the depth of implantation. (C) Pictorial diagram demonstrating depth of implantation (double-headed arrows).
As a result of our previous work relating depth of implantation to the development of conduction abnormalities, we now aim to position the ventricular edge of the prosthesis approximately 6-7 mm below the lower edge of the non-coronary leaflet. In an effort to assess the clinical impact of our previous findings1, we also compared the first half and second half of patients with respect to the depth of implantation from the non-coronary sinus.
The percent expansion of the inflow portion of the frame was defined as the diameter of the inflow portion of the frame measured by quantitative techniques divided by the nominal diameter of the inflow portion of the frame (either 26 or 29 mm depending on the size of the valve implanted). This was calculated in two orthogonal views using biplane imaging. We wanted to investigate the association between the actual expansion of the inflow portion of the frame and newly acquired conduction abnormalities.
Statistical analysis
Continuous variables are presented as means (±SD) or median (interquartile range, IQR) depending if the data is normally or non-normally distributed, respectively. Categorical variables are presented as frequencies. The Wilcoxon signed-rank test (for continuous variable) and the McNemar test conducted by exact methods (for binomial variables) were used to perform paired comparisons between pre-treatment vs. after treatment, pre-treatment vs. 1-month follow-up, pre-treatment vs. 6-month follow-up, after treatment vs. 1-month follow-up, after treatment vs. 6-month follow-up, and 1-month vs. 6-month follow-up. The analysis was performed upon the entire cohort that included 89 available ECGs before treatment, 85 after treatment, 47 at 1-month follow-up, and 45 at 6-month follow-up. To verify the consistency of the results, the analysis was repeated upon a cohort of 44 patients who had complete serial ECG follow-up.
We used a univariable logistic regression model to examine for separate predictors of new-onset left bundle branch block acquired after valve implantation (as opposed to new-onset left bundle branch block acquired before the valve was implanted) or new permanent pacemaking. Patients with pre-existing LBBB or pre-existing permanent pacemakers were excluded from the respective analyses. The following variables were included in the univariable analysis: baseline characteristics listed in Table 1, conduction abnormalities identified on the baseline ECG interpreted from Table 2, septal wall thickness (mm), the size of implanted valve (26 or 29 mm), ratio of the size of implanted valve to the diameter of the aortic valve annulus, diameter of the inflow portion of the frame (in AP and lateral projections), depth of implantation of the frame (from the non-coronary and left coronary view) and post-implant dilatation.

Statistical tests were two-sided with a p-value of < 0.05 indicating statistical significance. All statistical analyses were performed with SPSS software version 12 (SPSS Institute, Chicago, IL, USA).
Results
We prospectively enrolled 91 consecutive patients in whom a CoreValve ReValving System was implanted between November 2005 and April 2009. Baseline characteristics are listed in Table 1.
As a result of three intra-procedural deaths, procedural success was achieved in 88/91 patients (97%). Median duration of follow-up was 213 days (IQR 64, 519). Cumulative survival at 30 days and six months was 87% and 83%, respectively. Survival data was 100% complete at 1- and 6-month follow-up.
At 6-month follow-up, 28/91 patients (31%) had died. Sudden cardiac death (SCD) was responsible for 5/28 deaths (18%); these occurred on day 8, 244, 282, 400 and 887. In these patients, the latest electrocardiographic findings demonstrated normal sinus rhythm in one, incomplete left bundle branch block in one, left bundle branch in two, and a paced rhythm in one patient.
Twelve-lead ECG evaluation
The number of 12-lead ECGs analysed at each follow-up interval is shown in Figure 2.
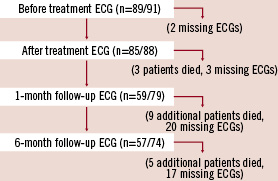
Figure 2. Patient flow diagram showing number of 12-lead electrocardiograms (ECGs) available for interpretation.
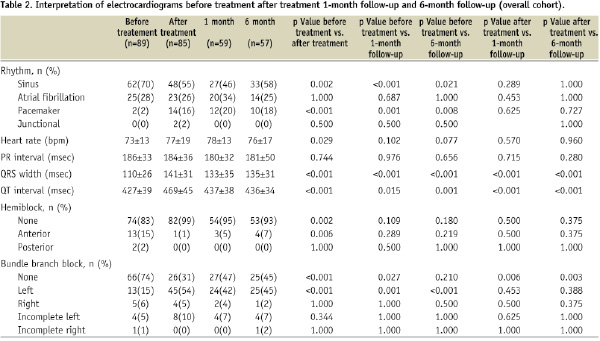
Results of the ECG interpretation are shown in Table 2 for the total cohort.
RHYTHM
The percent of patients in sinus rhythm decreased significantly from 70% before treatment to 55% after treatment (p=0.002). This decrease remained significant at 1- and 6-month follow-up (70 vs. 44%, p < 0.001 and 70 vs. 58%, p=0.021, respectively).
There was a significant increase in the frequency of paced rhythm after device insertion (2% before vs. 16% after treatment, p <0.001) that was maintained at 1- and 6-month follow-up (2% before vs. 21% at 1-month follow-up, p=0.004, and 2% before vs. 18% at 6-month follow-up, p=0.031).
QRS DURATION
After device insertion, there was a significant increase in QRS duration (110±26 before treatment vs. 141±31 msec after treatment, p <0.001). Although the QRS duration decreased significantly from after treatment to 1- and 6-month follow-up (141±31 after treatment vs. 131±29 msec at 1-month follow-up, p <0.001 and 141±31 after treatment vs. 134±30 msec at 6-month follow-up, p < 0.001, respectively), there was no significant change between 1 and 6-month follow-up (131±29 at 1-month follow-up vs. 134±30 msec at 6-month follow-up, p=0.79).
Analysis of individual patient data from 1- to 6-month follow-up demonstrated that 45% had a decrease in QRS width, 19% had no change, and 36% had an increase. The range for the differences in QRS width from 1- to 6-month follow-up was –52 to 58 msec.
BUNDLE BRANCH BLOCK
New-onset left bundle branch block (LBBB) occurred in 38% of patients after device insertion (15% before treatment vs. 53% after treatment, p <0.001). The number of patients with new-onset LBBB remained significantly higher at 1- and 6-month follow-up than at baseline (15% before treatment vs. 44% at 1-month follow-up, p=0.004, and 15% before treatment vs. 43% at 6-month follow-up, p=0.001, respectively). Furthermore, there was no significant change in the frequency of LBBB from after treatment to 1- or 6-month follow-up (53% after treatment vs. 44% at 1-month follow-up, p=1.0, and 53% after treatment vs. 43% at 1-month follow-up, p=0.45).
SERIAL ECG FOLLOW-UP IN 44 PATIENTS
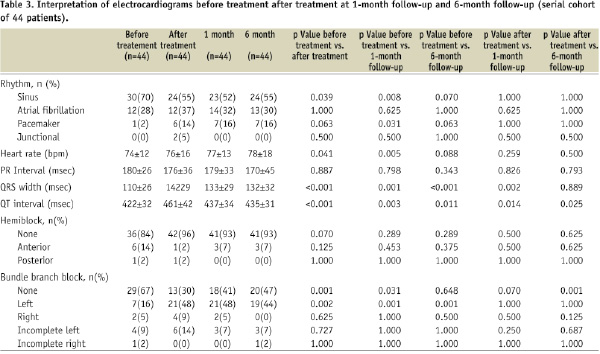
Similar results to those of the entire cohort (Table 2) were observed in 44 patients with complete serial ECG follow-up (Table 3).
TIMING OF QRS WIDENING
Examination of the intra-procedural 3-lead rhythm strip revealed that QRS widening occurred before and after valve implantation in 48% and 52% of patients, respectively. In those cases occurring before valve implantation, there was a temporal relation with either wire crossing of the native aortic valve or pre-implantation balloon aortic valvuloplasty.
NEED FOR PACEMAKING
Approximately one-tenth of patients had a history permanent pacemaker implantation before valve insertion (Table 1). During the procedure, the need for temporary pacemaking was documented in 19/91 patients (21%). Of those with a requirement for temporary pacemaking, 13/19 patients (68%) ultimately received a permanent pacemaker.
There were 17 patients who ultimately received a new permanent pacemaker. Considering the fact that there were three intra-procedural deaths and eight patients with a prior history of permanent pacemaker, 17/80 patients (21%) had a requirement for new permanent pacemaking. Thus, 13/17 patients (76%) implanted with a permanent pacemaker had a documentation of temporary pacemaking during the procedure. Third degree atrioventricular block was the indication for permanent pacemaker implantation in 14/17 patients (82%). The permanent pacemaker was implanted within seven days of the index procedure in 8/17 patients (47%), within 7-30 days in 6/17 patients (35%), and after 30 days in 3/17 (18%) (Table 4).
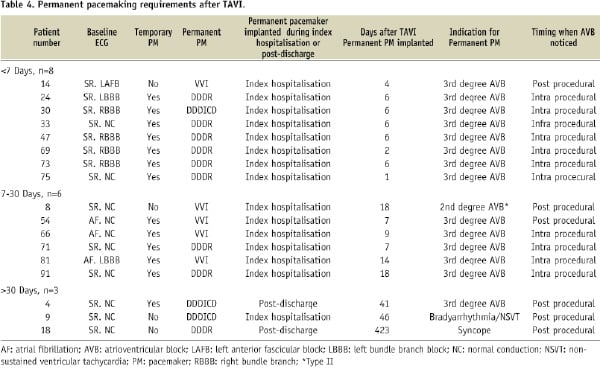
More precisely, the median time from valve to permanent pacemaker implantation was seven days (IQR 6, 18 days). Furthermore, the vast majority of patients (88%) had the permanent pacemaker implanted during their hospitalisation. The median length of hospital stay was significantly longer in patients implanted with a new permanent pacemaker than in those not implanted with a permanent pacemaker (17 IQR 9, 21 days vs. 9 IQR 7, 12 days, p=0.015).
All five patients with a pre-existing right bundle branch block developed complete heart block after device insertion.
QUANTITATIVE ANGIOGRAPHIC MEASUREMENTS
Measurements of the depth of implantation of the frame (mm) and diameter of the inflow portion of the frame (percent expansion and difference between nominal (26 or 29 mm inflow) and actual measurements) are shown in Table 5.
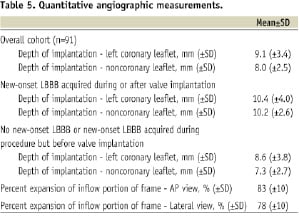
The depth of implantation measured from the non-coronary sinus was significantly greater in patients who developed LBBB after the valve was implanted than in those who did not develop LBBB or did develop LBBB but before the valve was implanted (7.3±2.7 vs. 10.2±2.6 mm, p<0.001); there was no difference in depth of implantation relative to the left coronary sinus (8.6±3.8 vs. 10.4±4.0 mm, p=0.106). Figure 3 shows the positive correlation between the depth of implantation and percent expansion of the inflow portion of the frame and its relationship to the development of LBBB after valve implantation.
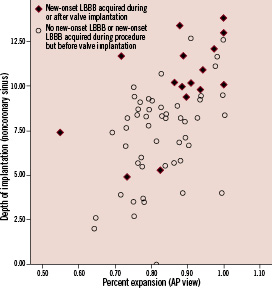
Figure 3. Correlation between depth of implantation (from the non-coronary sinus) and percent expansion of inflow level of the CoreValve (relative to valve size 26- or 29-mm). Pearson Correlation Coefficient 0.51, p<0.001.
Despite our intention to implant the valve at a target depth of 6-7 mm, we did not find a significant difference in the depth of implantation between the first- and second-half of the study patients (8.2±2.9 mm vs. 7.8±2.9 mm, respectively, p=0.56).
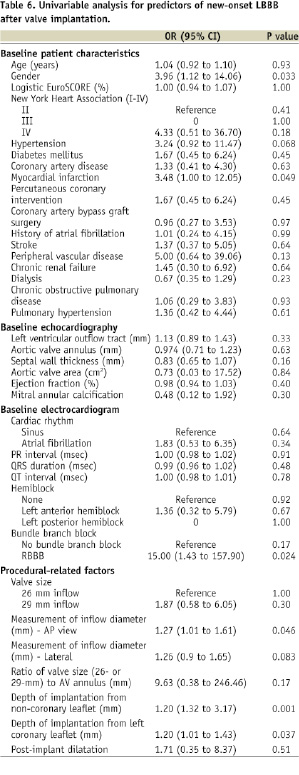
PREDICTORS OF NEW-ONSET LEFT BUNDLE BRANCH BLOCK AND NEED FOR PERMANENT PACEMAKING
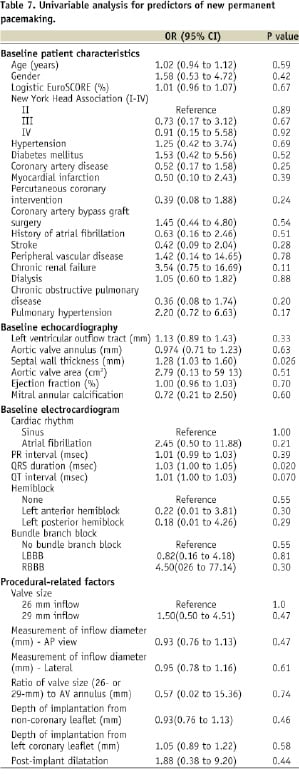
Tables 6 and 7 show the results of the univariable analysis for predictors of new-onset LBBB and need for new permanent pacemaking, respectively.
Discussion
In this study we found that early conduction abnormalities (LBBB) occurring after CoreValve implantation persisted at 6-month follow-up. In addition, one-fifth of patients had implantation of a new permanent pacemaker –50% were implanted seven days after the TAVI procedure. Predictors of new-onset LBBB were male gender, previous history of myocardial infarction, pre-existing right bundle branch block, actual diameter of the inflow portion of the frame after implantation and depth of implantation. Predictors for new permanent pacemaking were pre-treatment QRS duration (msec) and septal wall thickness. Finally, we propose a framework to understand the potential mechanisms implicated in the development of conduction abnormalities after TAVI.
We have previously demonstrated that new-onset LBBB is the most common conduction abnormality after implantation of the CoreValve device and is persistent at 1-month follow-up1. These findings have been confirmed by subsequent studies3,4. In our previous study, however, the observed decrease in QRS duration at 1-month follow-up (150±32 msec after treatment vs. 134±29 msec at 1-month follow-up, p<0.001) would seem to imply an improvement in conduction1. This led us to question whether conduction abnormalities occurring after CoreValve implantation were transient or persistent? Possible explanations include inflammation, oedema, ischaemia and/or mechanical stress with recovery of conduction. In the present study, we observed a significant decrease in QRS duration from after treatment to 1-month follow-up (143±31 vs. 132±30 msec, p=0.002) but there was no significant change in QRS duration or frequency of LBBB from 1-month to 6-month follow-up. In contrast, Gutiérrez et al observed a significant decrease in QRS duration (msec) and frequency of new-onset LBBB from post-treatment to 1-month follow-up after implantation of the balloon-expandable Edwards prosthesis (Edwards Lifesciences, Irvine, CA, USA) such that there was no significant difference between baseline and 1-month follow-up (LBBB pre-treatment 9% vs. post-treatment 27% vs. 1-month follow-up 13% and QRS duration pre-treatment 114 vs. post-treatment 129 vs. 1-month follow-up 118 msec). We speculate that differences in QRS duration and new-onset LBBB overtime between the self-expandable and balloon-expandable transcatheter aortic valve systems may be related to technical differences (e.g., depth of implantation) and physical design properties of the frame and/or stent (self-expanding nitinol vs. balloon expandable stainless steel).
The implications of persistent LBBB after TAVI are currently unknown. What is known, however, is that acquirement of new bundle branch block after surgical aortic valve replacement is associated with increased risk of subsequent arrhythmic events after 1-year follow-up (specifically, syncope, AV dissociation, and sudden death)8,13. Our study was underpowered to pursue a similar analysis. These surgical reports propose prophylactic pacemaker insertion in patients who develop bundle branch block after aortic valve replacement8,13.
In our centre, we do not implant “prophylactic” permanent pacemakers (e.g., for new-onset LBBB). The concept of “prophylactic” permanent pacemaker stems from the surgical literature. In a study of 133 patients undergoing surgical aortic valve replacement, Thomas et al observed that 32% of patients developed new-onset LBBB. At 2.5 years mean follow-up, the cumulative survival was 24% lower in those who developed new onset LBBB than in those who did not develop LBBB (66% vs. 90%, p<0.001) (reference). In a similar study of 389 patients undergoing surgical aortic valve replacement, El-Khally et al observed that 16% of patients developed a new-onset bundle branch block. At a mean follow-up of 4.5 years, the composite endpoint of complete heart block, syncope, and sudden cardiac death was 16% higher in those patients who developed bundle branch block than in those who did not develop bundle branch block. Both investigators concluded that “prophylactic permanent pacemaker” should be considered in patients who develop bundle branch block after surgical aortic valve replacement.
A new permanent pacemaker was inserted in approximately one-fifth of our patients after CoreValve implantation. The higher rate of permanent pacemaker implantation reported by other centres (up to 33%) most likely reflects a combination of “prophylactic” pacemaker implantation for concerns of patient safety and administrative logistics4,14. In our study, 3rd AV block was present in four-fifths of patients who received a new permanent pacemaker. Although 15/17 patients (88%) underwent permanent pacemaker implantation during hospitalisation, 9/17 (52%) patients underwent implantation seven days after valve implantation. The duration of hospitalisation was significantly longer in those patients who required permanent pacemaking than in those who did not require permanent pacemaking. Strategies to avoid unnecessary and prolonged hospitalisation due to pacemaker implantation should be the focus of future studies. Considering the fact that three-quarter of patients requiring temporary pacemaking ultimately required permanent pacemaking, immediate implantation of a permanent pacemaker in these particular patients may promote earlier mobilisation and discharge. In fact, anecdotal experiences seem to suggest that “prophylactic” permanent pacemaker implantation may conceivably lead to reduced hospital stay.
In the present study male gender, previous myocardial infarction, pre-existing right bundle branch block, diameter (mm) of the inflow portion of the frame after implantation, and depth of implantation were predictors for new-onset left bundle branch block.
Recently, Osamu et al examined the distance (mm) from the base of the non-coronary cusp to the lower edge of the membranous septum where the left bundle branch originated invariably in 100 autopsied hearts15. More specifically, the left bundle branch was located 6.3±2.4 mm from the base of the non-coronary cusp. Thus it is highly plausible that a deep implantation of the CoreValve device might play an important role in the occurrence of LBBB. The depth of the left bundle branch as noted by Osamu et al appears to be consistent with our suggestion to implant the CoreValve device <6-7 mm from the basal attachment of the aortic valve leaflets.
Two recent studies support our findings about the correlation between the depth of implantation and occurrence of LBBB after transcatheter aortic valve implantation. Gutiérrez et al observed a correlation between the depth of implantation of the Edwards SAPIEN (Edwards Lifesciences) device and the occurrence of left bundle branch block16. More specifically, 35% of patients in whom the ventricular end of the prosthesis was located below the hinge point of the anterior mitral leaflet developed left bundle branch block compared with none of the patients in whom the ventricular end was implanted above the hinge point (identified by transesophageal echocardiography) (p=0.029). In another study, Koektuerk et al found a correlation between the depth of implantation (>8 mm) of the CoreValve device assessed by fluoroscopy and the need for permanent pacemaking17.
Despite our intentions to implant the prosthesis at a target depth of 6-7 mm, we did not find a significant difference in the depth of implantation between the first and second half of the study patients. This observation may question the technical feasibility (whether device- or operated-related) to precisely implant the CoreValve at a particular location. It is possible the learning curve and iterations in device design will improve the accuracy of device implantation.
In the present study, pre-treatment QRS duration and septal wall thickness were univariate predictors for permanent pacemaking. This would imply that patients with pre-existing conduction disease would have a greater chance of receiving a permanent pacemaker after CoreValve implantation. A single-centre study by Jilaihawi et al also identified septal wall thickness, in addition to LBBB with left axis deviation and non-coronary cusp thickness >8 mm as predictors for permanent pacemaking after CoreValve implantation4. Independent predictors for permanent pacemaking after surgical aortic valve replacement include age, pre-existing right and left bundle branch block, aortic regurgitation at baseline, multivalvar surgery, left atrial enlargement, left ventricular dysfunction, pulmonary hypertension, previous myocardial infarction, and post-operative electrolyte imbalances18-21.
As can be appreciated from Table 4, there was a discrepancy between the timing “when the AV block was first noticed” and “when the permanent pacemaker was implanted”. Retrospective analysis of the patient dossiers revealed that the disparity between recognising the AV block and implantation of the permanent pacemaker was mainly due to administrative logistics and in some patients, urinary tract infection or low-grade fever of unknown origin that eventually resolved. Of the 15 patients who had a permanent pacemaker implanted during the index hospitalisation, AV block was recognised during the procedure in 11 patients (73%) and post-procedure in four patients (27%).
We propose the following framework to better understand the potential mechanisms involved in the development of conduction abnormalities: (1) patient-related; (2) anatomical-related; and (3) procedural-related (e.g., device or operator-related). Thus, gender, previous myocardial infarction and pre-existing right bundle branch block can be considered as patient-related factors; variations in the location of the left bundle branch exit point as an anatomical-related factor; self-expanding characteristics and high radial force of the CoreValve frame as device-related; and control of the depth of implantation as operator-related.
Study limitations
The following limitations need to be addressed. This report represents a single centre experience and therefore the results may not be generalisable. The results of this study relate to only one type of prosthesis and the conclusions, therefore, may not be applicable to other transcatheter aortic valve bioprostheses. Furthermore, variables (e.g., degree of aortic valve calcification) potentially associated with development of conduction abnormalities after CoreValve implantation may have been omitted from the analysis. The modest number of patients included in the analysis may have been underpowered to identify additional predictors of new LBBB or need for permanent pacemaking.
Currently, there is no gold standard to measure the depth of implantation of transcatheter aortic valves. In this study, the depth of implantation was measured by quantitative angiographic measurements using the “working” gantry angle (i.e., implantation viewing angle). The possibility exists that the depth of implantation was systematically underestimated. The alternative of overestimating the depth of implantation is not possible. Nevertheless, it was in this “working” gantry angle that the operators estimated the depth of implantation during the procedure. Also of note is that there was minimal overlap in the depth of implantation between those with new-onset LBBB vs. those who did not develop LBBB (10±2 mm vs. 7±2 mm, respectively). Alternative imaging modalities such as MSCT or echocardiography were not used to assess the depth of implantation and may have provided additional information.
This study was not designed to systematically interrogate newly implanted pacemakers at 1- and/or 6-months. It is possible that complete AV conduction disturbances may disappear as a sign of healing after some days or weeks.
Practical clinical implications
Our results would suggest that implanting the CoreValve prosthesis in a more superior location within the left ventricular outflow tract (i.e., depth of implantation <6-7 mm) might mitigate the occurrence of LBBB. It is likely that a number of inter-relating factors such as patient characteristics, anatomic variations, operator technique, and device characteristics are playing a role in the development of conduction abnormalities after TAVI. The clinical consequences of new-onset LBBB or new permanent pacemaking after CoreValve implantation are currently unknown.
The significant inter-hospital variations in the frequency of new permanent pacemaker reported in the literature likely reflects variations in physician threshold, country-based healthcare norms, and reimbursement strategies. There is currently a lack of standardised guidelines for new permanent pacemaker implantation, duration of temporary pacemaking, and duration of monitoring (in-hospital or post-discharge) after TAVI. In our centre, we use the AHA/ACC guidelines on when to implant a permanent pacemaker after TAVI22. The temporary pacemaker wire is typically removed on day 2-3 post-procedure. Subsequently, patients are maintained on telemetry monitoring for the duration of hospitalisation. An ECG is obtained at 1-month, 6-month, and 12-month clinical follow-up.

