
Abstract
Aims: Conventional aortic valve replacement (AVR), sutureless AVR (su-AVR) and transcatheter aortic valve implantation (TAVI) for severe aortic stenosis (AS) are associated with conduction abnormalities. The aim of the present study was to assess the incidence of left bundle branch block (LBBB) after su-AVR and TAVI, in comparison to conventional AVR.
Methods and results: A total of 501 patients (mean age 74±8 years, 53% male) without preoperative cardiac conduction disturbances who underwent AVR or TAVI were included in the study. Su-AVR patients and TAVI patients had a higher incidence of new-onset LBBB at hospital discharge (23% and 16%, respectively) compared to patients treated with conventional AVR (4%; p<0.001). On multivariate logistic regression analyses, the type of AVR was independently associated with complete LBBB, after correcting for age, preoperative QRS duration and heart rate (su-AVR and TAVI relative to the reference category conventional AVR: odds ratio [OR] 8.5, 95% confidence interval [CI]: 3.7-19.5; p<0.001, and OR 5.8, 95% CI: 2.4-14.1; p<0.001, respectively).
Conclusions: Su-AVR and TAVI were associated with higher risk of developing postoperative LBBB compared to conventional AVR, after adjusting for age, preoperative heart rate and QRS duration.
Introduction
Severe aortic stenosis (AS) is the most prevalent valvular heart disease among elderly populations1. The selection of the type of aortic valve replacement (AVR, surgical versus transcatheter) and the type of prosthesis (biological versus mechanical) depends on the clinical characteristics and operative risks of the patients2. Particularly in the subgroup of elderly patients with symptomatic severe AS, a bioprosthesis is preferred over a mechanical prosthesis in order to minimise the risks of bleeding associated with lifelong anticoagulation treatment3. The advent of transcatheter aortic valve implantation (TAVI) and the development of sutureless biological prostheses have expanded the therapeutic alternatives in elderly patients with symptomatic severe AS. In high surgical risk patients, several studies have shown comparable midterm outcomes of transcatheter bioprostheses, sutureless bioprostheses and stented bioprostheses4,5. One of the complications that may occur after TAVI and surgical AVR is new-onset left bundle branch block (LBBB)6. However, there is a wide range in the reported incidences of new-onset LBBB which may be explained by the presence of pre-existing conduction disturbances, the position of the prosthesis in the left ventricular (LV) outflow tract and the type of prosthesis7-10. The aim of the present study was to assess the incidence and factors associated with the development of LBBB after AVR using a sutureless prosthesis (su-AVR) and after TAVI, in comparison to conventional surgical AVR.
Methods
PATIENTS
Of 682 patients who underwent AVR from 2008 to 2014 at the Leiden University Medical Center (The Netherlands), 501 were considered eligible for enrolment in the study based on analysable preoperative and postoperative electrocardiograms (ECG) and preoperative transthoracic echocardiography.
Patients were divided into three groups based on the treatment: su-AVR, TAVI or conventional AVR (Figure 1). Clinical characteristics were prospectively collected in the departmental Cardiology Information System (EPD-Vision®; Leiden University Medical Center, Leiden, The Netherlands) and retrospectively analysed. The institutional review board approved this retrospective analysis of clinically acquired data and waived the need for patient written informed consent.
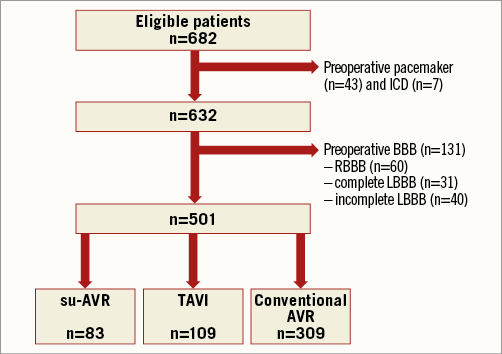
Figure 1. Flow chart of patient inclusion. AVR: aortic valve replacement; BBB: bundle branch block; ICD: implantable cardioverter defibrillator; LBBB: left bundle branch block; RBBB: right bundle branch block; su-AVR: sutureless aortic valve replacement; TAVI: transcatheter aortic valve implantation
ELECTROCARDIOGRAPHY
Standard 12-lead ECGs were obtained before and after AVR at the day of hospital discharge. Heart rate, rhythm, axis, QRS duration and the presence of bundle branch block were assessed. Right bundle branch block (RBBB) was defined as a QRS duration >120 ms in the presence of typical RBBB morphology (rR’ in V1). LBBB was defined as QRS duration >120 ms and QRS complex negative in V1 with a small R or no R. Strict criteria were applied to define complete LBBB (QRS >140 ms in males and >130 ms in females with slurring or notching visible in at least two of the following leads: V1, V2, V5, V6, I and aVL)11.
TWO-DIMENSIONAL TRANSTHORACIC ECHOCARDIOGRAPHY
Preoperative transthoracic echocardiography was performed using commercially available ultrasound systems (Vingmed System FiVe, Vivid™ 7, and Vivid™ E9; General Electric Healthcare, Vingmed, Horten, Norway) equipped with 3.5 MHz or M5S-D transducers. Parasternal, apical, subcostal and suprasternal views were obtained according to current recommendations12. The echocardiographic data were digitally stored in cine-loop format and data were retrospectively analysed using commercially available software (EchoPac™ 112.0.1; GE Medical Systems, Horten, Norway). Left ventricular (LV) dimensions and ejection fraction (LVEF) were assessed as recommended12,13. Preoperative aortic valve function was evaluated using colour Doppler, continuous and pulsed wave Doppler according to current recommendations14,15.
SURGICAL AND TRANSCATHETER AORTIC VALVE REPLACEMENT
Treatment of aortic stenosis (surgical versus transcatheter) was decided based upon Heart Team discussions. Among patients who underwent surgical AVR, only patients who received a stented bioprosthesis were selected in order to minimise heterogeneity and to ensure comparable groups in terms of number of patients. Su-AVR was performed as previously described with a Perceval™ S valve (Sorin Biomedica Cardio Srl [now LivaNova], Sallugia, Italy) or 3f Enable™ valve (Medtronic, Minneapolis, MN, USA)16.
TAVI was performed according to current recommendations17. Only patients who underwent TAVI via the transfemoral approach were included to minimise heterogeneity. Balloon valvuloplasty was performed under rapid right ventricular pacing prior to transfemoral implantation of a balloon-expandable prosthesis (SAPIEN; Edwards Lifesciences, Irvine, CA, USA) or self-expanding prosthesis (CoreValve®; Medtronic, Minneapolis, MN, USA). Figure 2 shows schematically the position of the different implanted prostheses in relation to the conduction system, in particular the left bundle branch.
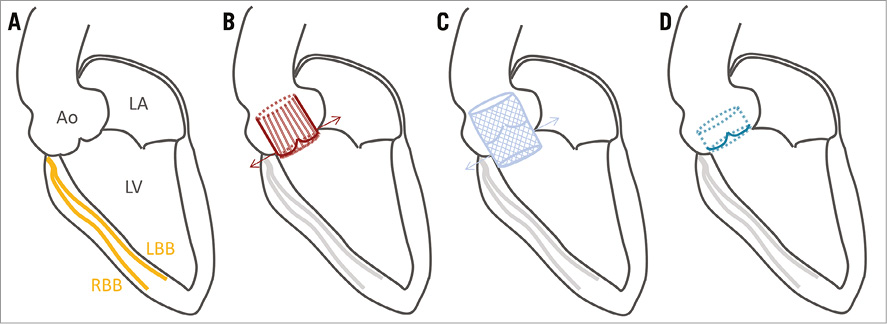
Figure 2. Schematic overview of prosthesis implantation. A) A 3-chamber view with the bundle branches. The su-AVR prosthesis (B) and TAVI prosthesis (C) were placed intra-annular. The conventional stented AVR prosthesis (D) was placed supra-annular. Ao: aorta; LA: left atrium; LBB: left bundle branch; LV: left ventricle; RBB: right bundle branch
STATISTICAL ANALYSES
All data analyses were performed using the SPSS software, Version 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables were reported as mean±standard deviation or median and interquartile range, as appropriate. Categorical variables were reported as counts and percentages. Differences were analysed using ANOVA or Kruskal-Wallis tests and the chi-square test.
Linear mixed model analysis was performed to compare changes in heart rate and QRS duration over time among the three groups. Type of surgery and time of ECG were incorporated in the model as fixed variables. An unstructured covariance matrix was applied. The estimated marginal means±standard error of the mean were presented.
Logistic regression analysis was performed to assess baseline factors associated with postoperative complete LBBB. All variables with a p-value <0.1 on univariate logistic regression analysis were included in the multivariate model. The odds ratio and 95% confidence interval were calculated. All statistical tests were two-sided. A p-value <0.05 was considered statistically significant.
Results
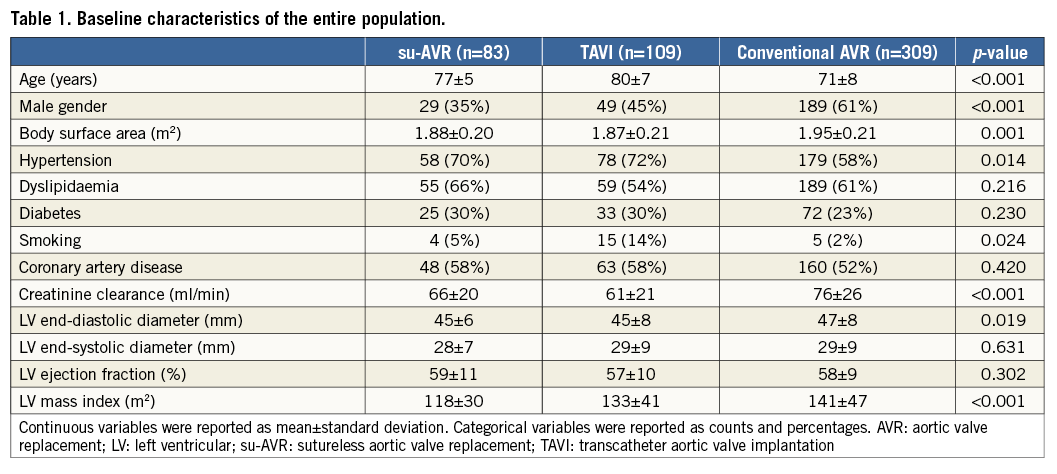
A total of 501 patients (mean age 74±8 years, 53% male) were included in the present analysis. Patients who underwent conventional AVR were significantly younger and more often male compared to su-AVR and TAVI groups. Preoperative characteristics are shown in Table 1.
SURGICAL CHARACTERISTICS
Su-AVR was performed with the 3f Enable valve in 68 patients (82%) and with the Perceval S valve in 15 patients (18%). TAVI was performed in 86 patients (79%) with a SAPIEN valve and in 23 patients (21%) with a CoreValve. Conventional AVR was performed with stented bioprostheses: Carpentier-Edwards Perimount Magna valve (Edwards Lifesciences) in 111 patients (36%), the Hancock valve (Medtronic) in 182 patients (59%) and the Trifecta™ valve (St. Jude Medical, St. Paul, MN, USA) in the remaining 16 patients (5%). The size of the prosthesis was significantly different among the three surgical techniques, with larger sizes among TAVI patients (23.5±2.0 mm in su-AVR, 25.9±2.2 mm in TAVI and 24.5±1.8 mm in conventional AVR; p<0.001).
ECG CHANGES AFTER AVR
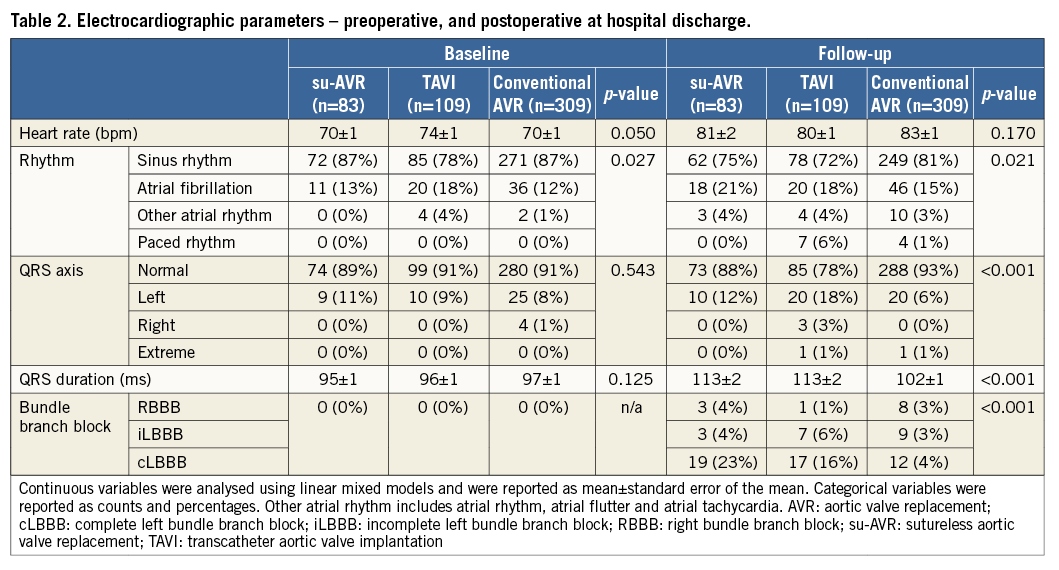
Table 2 shows the ECG parameters preoperatively and postoperatively at hospital discharge. Postoperative ECG was performed three days (interquartile range [IQR]: two to four days) after TAVI compared to six days (IQR: five to eight days) after su-AVR and six days (IQR: five to eight days) after conventional AVR (p<0.001). The heart rate increased significantly after intervention in all three groups. The majority of patients were in sinus rhythm (85%) before surgery. After surgery, the percentage of patients with atrial fibrillation increased to 17%, 17 patients showed atrial arrhythmia or junctional rhythm, and 11 patients showed paced rhythm.
The QRS duration increased significantly in all three groups after AVR. In addition, at hospital discharge, the QRS duration differed significantly among groups. Postoperatively, complete LBBB was observed significantly more often after su-AVR (23%) and TAVI (16%) compared to conventional AVR (4%; p<0.001). In addition, there were in total 12 patients (2%) with an RBBB and 19 patients (4%) with incomplete LBBB.
FACTORS ASSOCIATED WITH COMPLETE LBBB
The type of AVR was significantly associated with complete LBBB on univariate logistic regression analysis. Su-AVR (odds ratio [OR] 8.5, 95% confidence interval [CI]: 3.7-19.5; p<0.001) and TAVI (OR 5.8, 95% CI: 2.4-14.1; p<0.001) were independently associated with complete LBBB, after adjusting for age, preoperative rhythm and preoperative QRS duration.
Discussion
The main findings of the present study can be summarised as follows: at discharge, the incidence of LBBB was 23% after su-AVR and 16% in TAVI patients, compared to 4% in patients undergoing conventional AVR. Su-AVR patients and TAVI patients more often showed new-onset complete LBBB, in comparison to patients treated with conventional AVR.
INCIDENCE OF LBBB AFTER AVR
The incidence of new-onset LBBB after AVR ranged between 4 and 51% in previous studies9,10. Differences in the incidence of LBBB can be explained by differences in the type of procedure, valve type and follow-up duration. In conventional AVR with a stented bioprosthesis, the incidence of LBBB at hospital discharge was low (4-6%)10,18,19. In contrast, studies reporting the incidence of LBBB after su-AVR or TAVI have shown considerably higher incidences compared with conventional AVR (39% and about 21%, respectively)7-9,20-23. In TAVI, the type of valve was an important determinant of new-onset LBBB: the CoreValve was associated with a higher incidence of LBBB (48-51%) compared with the SAPIEN valve (12-27%)9,21. The present study showed a higher incidence of LBBB in TAVI with the CoreValve (22%) compared to the SAPIEN valve (14%), although this was not statistically significant (p=0.555). Studies with longer follow-up duration demonstrated that new-onset LBBB present at hospital discharge was transient in some cases and resolved after months of follow-up. Persistent LBBB was present in only 9% of TAVI patients and 2% of patients treated with conventional AVR8,10. Local inflammation, oedema and ischaemia of the surrounding tissue following aortic valve implantation may explain the transient nature of acute postoperative LBBB24.
MECHANISM UNDERLYING AVR-INDUCED LBBB
New-onset LBBB after AVR can be related to compression by the prosthesis on the conduction system. The bundle branch initiates at the base of the interleaflet triangle between the non-coronary and right coronary cusps, located at the aortic annulus6. Stented biological prostheses are placed supra-annular, whereas the su-AVR and TAVI prostheses are placed intra-annular, close to the bundle branch, which may lead to an increased risk of damage of the conduction system. Previous studies in TAVI patients showed that a lower implantation depth was associated with new-onset LBBB25,26.
Besides the position of the valve, the size of the implanted prosthesis relative to the annulus size is important in the pathophysiology of conduction abnormalities. In su-AVR and TAVI, slight oversizing is necessary to prevent severe paravalvular leakage27. However, excessive oversizing can result in increased compression of the conduction system and aortic annulus rupture28.
Another factor responsible for the occurrence of LBBB after AVR might be related to the expandable property of the su-AVR and TAVI prostheses. The stented biological prostheses are sutured to the annulus and afterwards the prosthesis does not generate a radial force that compresses the conduction system. In contrast, the su-AVR and TAVI prostheses are anchored into the aortic annulus and generate a radial force expansion that may compress the conduction system and lead to conduction abnormalities. Previous studies hypothesised that the nitinol frame of the CoreValve, with the unique property of shape memory, is responsible for increased ongoing compression on the conduction system, resulting in more frequent LBBB in comparison to the SAPIEN valve28. This may additionally explain the higher incidence of LBBB in su-AVR prostheses mounted in a nitinol frame. However, in a direct comparison between the CoreValve (with nitinol frame) and the SAPIEN XT (with a cobalt-chromium frame), there was no significant difference in the force posed on the annulus29.
CLINICAL IMPLICATIONS
The present study showed a significantly higher incidence of new-onset LBBB after su-AVR and TAVI in comparison to conventional AVR. The present results may impact on the selection of the type of surgery, especially in patients with an intermediate surgical risk. Furthermore, attention should be paid to the sizing and positioning of the prostheses in su-AVR and TAVI to improve outcomes with lower incidences of LBBB. Further technical development of both TAVI valves and sutureless valves and careful implantation of the valve (not too deep into the LVOT) may help to reduce the incidence of LBBB. Additionally, further clinical research should elucidate whether the occurrence of LBBB is transient or persistent and whether it impacts on the postoperative LV systolic function. Particularly in older patients with preoperative reduced systolic function, this could be of importance when selecting the type of surgery. It might influence the need for cardiac resynchronisation therapy in patients with impaired LV function who develop LBBB after AVR. Future studies should analyse which patients are more at risk in developing persistent LBBB and what actions can prevent new-onset LBBB.
Study limitations
The present study was retrospective, with all the inherent limitations of such a study design. The ECG parameters were assessed preoperatively and at discharge, with strict criteria for complete LBBB11. However, the present study did not evaluate whether LBBB was persistent or not during long-term follow-up. Because patients were discharged earlier after TAVI, the duration between surgery and postoperative ECG at discharge was shortest in this group compared to su-AVR and conventional AVR, which may have resulted in an increased incidence of transient LBBB among TAVI patients. In addition, both the CoreValve and the SAPIEN valve were used in the TAVI cohort. The CoreValve tended to result in more complete LBBB than the SAPIEN valve; however, this difference was not statistically significant and therefore both types of valve were included in the present analysis. Results cannot be extrapolated to stentless biological and mechanical prostheses and non-transfemoral TAVI. Furthermore, the present study described the first series of su-AVR patients; therefore, the learning curve could be a contributing factor to the relatively high incidence of LBBB. The depth of implant of the three types of prosthesis was not systematically evaluated and therefore analyses on whether the depth of implant influenced the prevalence of new-onset LBBB could not be performed.
Conclusions
In conclusion, su-AVR and TAVI patients more frequently developed postoperative LBBB at discharge in comparison to patients treated with conventional stented AVR bioprostheses. Su-AVR and TAVI were associated with a higher risk of developing postoperative LBBB compared to conventional AVR, after adjusting for age, preoperative heart rate and QRS duration.
| Impact on daily practice The present study shows that the development of left bundle branch block is more prevalent after sutureless and transcatheter aortic valve replacement in comparison to regular aortic valve replacement. This should be taken into consideration in the selection of the type of surgery. Further research should elucidate whether the postoperative conduction disturbances are transient or persistent and whether they impact on the postoperative left ventricular systolic function. This might influence the need for cardiac resynchronisation therapy in patients with an impaired left ventricular function who develop left bundle branch block after aortic valve replacement. |
Funding
The Department of Cardiology (Leiden University Medical Center) has received grants from Biotronik, Medtronic and Boston Scientific Corporation.
Conflict of interest statement
V. Delgado has received a speaker’s fee from Abbott Vascular. The other authors have no conflicts of interest to declare.

