Abstract
Background: No detailed data on left bundle branch block (LBBB) and permanent pacemaker implantation (PPI) exist from randomised clinical trials comparing the ACURATE neo and CoreValve Evolut devices.
Aims: Our aim was to assess the incidence and impact of new LBBB and PPI with self-expanding prostheses from a powered randomised comparison.
Methods: From the SCOPE 2 trial, 648 patients with no previous pacemaker were analysed for PPI at 30 days, and 426 patients without previous LBBB were adopted for analysis of LBBB at 30 days.
Results: At 30 days, 16.5% of patients required PPI; rates were higher in CoreValve Evolut compared to ACURATE neo recipients (21.0% vs 12.3%; p=0.004). Previous right bundle branch block (odds ratio [OR] 6.11, 95% confidence interval [CI]: 3.19-11.73; p<0.001) was associated with an increased risk of PPI at 30 days, whereas the use of the ACURATE neo (OR 0.50, 95% CI: 0.31-0.81; p=0.005) was associated with a decreased risk. One-year mortality was similar in patients with and without new PPI. A total of 9.4% of patients developed persistent LBBB at 30 days, with higher incidences in CoreValve Evolut recipients (13.4% vs 5.5%; p=0.007). New LBBB at 30 days was associated with lower ejection fraction at 1 year (65.7%±11.0 vs 69.1%±7.6; p=0.041).
Conclusions: New LBBB and PPI rates were lower in ACURATE neo compared to CoreValve Evolut recipients. The ACURATE neo valve was associated with a lower risk of PPI at 30 days. No effect on 1-year mortality was determined for PPI at 30 days, while LBBB at 30 days was associated with reduced ejection fraction at 1 year.
Introduction
Transcatheter aortic valve replacement (TAVR) has developed from a therapeutic option initially reserved for inoperable and high-risk patients to an accepted alternative to surgery in intermediate- and low-risk patients12345. Through technological refinement and increased operator experience, complication rates have drastically reduced over the years; however, the development of post-operative conduction abnormalities, such as new left bundle branch block (LBBB) and higher degree conduction disturbances needing new permanent pacemaker implantation (PPI), have persisted as concerning complications6. While some studies showed no prognostic impact, recent investigations have attributed an increased risk of mortality or impaired recovery of left ventricular (LV) function789 to LBBB and PPI.
Rates of new LBBB and PPI differ considerably between transcatheter heart valves (THV); yet, to date, randomised comparative evidence remains scarce. The SecOnd-generation seLf-expandable Versus Balloon-expandable Valves and gEneral Versus Local Anesthesia in TAVI (SOLVE-TAVI) randomised trial showed a trend towards higher PPI rates in recipients of the CoreValve Evolut R (Medtronic) versus the SAPIEN 3 (Edwards Lifesciences)10, while the SCOPE I randomised trial showed similar PPI rates between the SAPIEN 3 and the ACURATE neo (Boston Scientific) THV11. The SCOPE 2 randomised trial was designed to compare the performance of the ACURATE neo and the CoreValve Evolut R THV and was appropriately powered to detect a difference in PPI rates among these THV at 30 days. The ACURATE neo THV was reported to have exhibited significantly lower PPI rates as compared to the CoreValve Evolut THV in this trial12.
Despite the existence of registry-based attempts to identify the predictors of new PPI and conduction disturbances after TAVR with the ACURATE neo and the CoreValve Evolut THV131415, solid evidence from prospective randomised controlled data with centrally adjudicated outcomes has remained an unmet clinical need.
In this non-prespecified subanalysis of the SCOPE 2 randomised trial, we aimed to i) assess independent predictors of new PPI after TAVR, focusing on clinical baseline characteristics, computed tomography (CT)-assessed valve morphology and pre-existing electrocardiographic variables; ii) assess whether newly developed conduction abnormalities resolve or persist from discharge to follow-up at 30 days and 1 year; iii) establish whether new LBBB or PPI after TAVR have an impact on mortality at 1 year between 2 contemporary self-expanding THV prostheses.
Methods
Study design and definition of endpoints
The SCOPE 2 trial was a multicentre, randomised, parallel design, non-inferiority, open-label trial conducted at 23 high-volume heart valve centres in Europe. Details of the trial design and study population have been previously described12. In short, eligible patients were randomly assigned in a 1:1 ratio to undergo TAVR with either the ACURATE neo THV or the CoreValve Evolut R THV and its later iterations. The primary endpoint was a composite of all-cause death or any stroke at 1 year powered for non-inferiority of the ACURATE neo THV, which was not met (absolute risk difference 1.8%, upper 1-sided 95% confidence limit: 6.1%; p=0.0549 for non-inferiority). The prespecified and powered key secondary endpoint was new PPI at 30 days. Additional secondary endpoints included the components of the primary endpoint at 30 days and 1 year, as well as, among others, the incidence of new LBBB. Endpoints were defined according to the Valve Academic Research Consortium 216 and an independent clinical events committee (Cardiovascular European Research Center [CERC], Massy, France) adjudicated all endpoint-related adverse events. All follow-up echocardiograms were assessed by an independent core laboratory (CERC).
For the purpose of this subanalysis from the SCOPE 2 trial, which was designed to specifically identify the predictors of new conduction abnormalities and PPI, an as-treated population from the SCOPE 2 database was adopted, considering the treatment actually received by the participants, regardless of their adherence to the randomisation assignment. Unlike the original analyses, which applied intention-to-treat (ITT) and per-protocol populations, the as-treated population was adopted to specifically evaluate THV-dependent endpoints. Furthermore, only patients who survived at 30 days or with known pacemaker status at 30 days were included and 2 study populations were defined: (i) to analyse the incidence and impact of new PPI at 30 days, patients with prior pacemakers were excluded, resulting in the designated PPI 30 cohort; and (ii) to analyse the incidence and impact of novel LBBB after TAVR, patients with missing or uninterpretable electrocardiogram (ECG) at baseline, discharge or 30 days, as well as patients with prior LBBB, were excluded. The remaining patients became the designated LBBB 30 cohort. A detailed study flow chart is depicted in Figure 1. New persistent LBBB at 30 days was defined as new-onset LBBB after TAVR, which persisted up to 30 days, while LBBB resolution on ECG at 30 days was considered transient LBBB. Annular eccentricity was assumed for an eccentricity index (EI)>0.25, calculated as: 1 − minimum diameter/maximum diameter1718.
Follow-up was conducted up to 1 year after TAVR and included the assessment of all-cause mortality, development of New York Heart Association (NYHA) Functional Class and left ventricular (LV) function on echocardiography. Approval from an appropriately constituted competent ethics committee was sought at each site, and the study conduct complied with the Declaration of Helsinki.
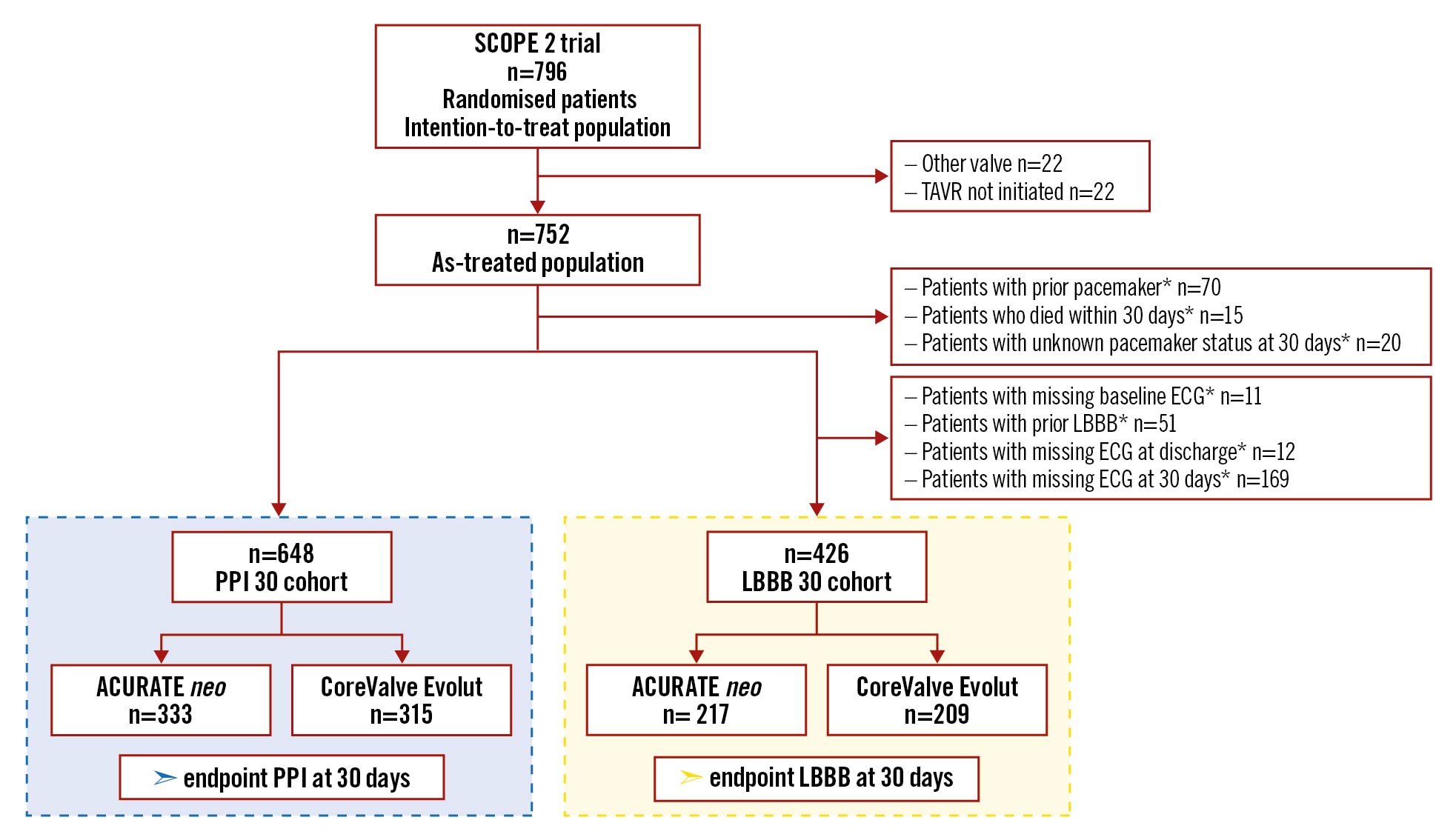
Figure 1. Study flow chart. Study flow chart showing exclusion criteria and the two adopted patient cohorts. *multiple events possible. ECG: electrocardiogram; LBBB: left bundle branch block; PPI: permanent pacemaker implantation; TAVR: transcatheter aortic valve replacement
Statistical analysis
Continuous variables are presented as mean with standard deviation (SD) or median with interquartile range (IQR), and were compared using the Student’s t-test or the Mann-Whitney U test, respectively. Categorical and ordinal variables are expressed as frequencies and proportions and were compared using the chi-square or Fisher’s exact tests. Nominal logistic regression with the computation of odds ratios (OR) and 95% confidence intervals (CI) was used to assess the association between the type of THV and need for PPI at 30 days. To avoid overfitting, the selection of covariates in the multivariable regression model was performed using the least absolute shrinkage and selection operator regression method after entering baseline and procedural characteristics with a potential effect on outcome as candidates. These included the use of the ACURATE neo THV, the logistic EuroSCORE, history of atrial fibrillation, complete right bundle branch block (RBBB) and LBBB at baseline, moderate-to-severe aortic valve and left ventricular outflow tract (LVOT) calcification, as well as pre- and post-dilatation. Observations with missing data were excluded. As an additional sensitivity analysis, the association between the type of THV and need for PPI at 30 days was analysed in the ITT population, as well as in a “modified PPI 30 cohort”. The latter included patients who died within 30 days (n=662). To explore the effect of THV on PPI at 30 days in subsets of patients, subgroup analyses were performed for patients with pre-existing RBBB, history of atrial fibrillation, small aortic annuli (defined as annulus perimeter ≤72 mm), eccentric annuli (defined as EI>0.25) and based on aortic valve and LVOT calcification (none/mild vs ≥moderate). For patients with known clinical status at 30 days, Kaplan-Meier survival curves, according to LBBB and PPI at 30 days, were computed for all-cause mortality during 1-year follow-up. A comparison of cumulative event rates between these groups was performed by the log-rank test. For the comparison of LV function during follow-up the Wilcoxon matched-pairs signed-rank test was applied.
A 2-sided p-value of <0.05 was considered statistically significant for all analyses. IBM SPSS Statistics (Version 27.0.1.0; IBM), JMP Version 13.0 software (SAS) and R (Version 4.0.3; The R Foundation) were used for statistical analyses.
Results
Patient population
A total of 796 patients were enrolled in the SCOPE 2 trial, 398 of which were allocated to the ACURATE neo and 398 to the CoreValve Evolut THV. Applying the above-mentioned exclusion criteria (detailed in Figure 1), 648 patients formed the PPI 30 cohort, 333 of which were treated with the ACURATE neo and 315 with the CoreValve Evolut THV. Furthermore, 426 patients formed the LBBB 30 cohort, 217 and 209 of which were treated with the ACURATE neo and the CoreValve Evolut THV, respectively.
Permanent pacemaker implantation - predictors and impact on outcome
Overall, 16.5% (107/648) of patients required a PPI at 30 days, 72.9% of which were implanted within 3 days from the TAVR procedure, while only 3 patients required PPI between 30 days and 1 year. A total of 79.4% of patients who required PPI at 30 days had a dual chamber device implanted, 17.8% had a single chamber device and 1.9% had a biventricular device (0.9% unknown). The indication for PPI at 30 days was a Mobitz type II atrioventricular (AV) block or a Mobitz type III AV block in the vast majority of patients, showing no significant difference between the ACURATE neo and CoreValve Evolut recipients (78.0% vs 75.8%; p=0.785). Infrequent indications, comprising LBBB, first degree atrioventricular block (AV block I) etc., are reported in Supplementary Table 1. Baseline characteristics of the PPI 30 cohort according to the implanted THV are depicted in Supplementary Table 2 and showed no significant differences, except for a higher rate of AV block I and larger aortic annulus perimeter for patients receiving the ACURATE neo compared to the CoreValve Evolut THV. Baseline characteristics according to need for PPI at 30 days are shown in Table 1: the only differences were higher rates of RBBB and moderate to severe aortic valve calcification, as well as lower rates of LBBB in patients who required PPI at 30 days. Pre- and post-dilatation strategy did not differ between patients with or without PPI at 30 days (Table 1).
The crude rate of new PPI at 30 days was 12.3% (41/333) with the ACURATE neo THV, which was significantly lower than with the CoreValve Evolut THV (21.0% [66/315]; p=0.004) (Central illustration). In a multivariable model, RBBB was associated with an increased risk (OR 6.11, 95% CI: 3.19-11.73; p<0.001) and use of the ACURATE neo with a decreased risk of PPI at 30 days (OR 0.50, 95% CI: 0.31-0.81; p=0.005) (Supplementary Table 3, Figure 2). A sensitivity analysis of the multivariable model in the ITT population confirmed RBBB to be associated with an increased risk (OR 4.63, 95% CI: 2.62-8.20; p<0.001) and use of the ACURATE neo with a decreased risk of PPI at 30 days (OR 0.56, 95% CI: 0.37-0.85; p=0.006) (Supplementary Table 4, Figure 2). Similar findings were obtained in the multivariable model of the “modified PPI 30 cohort”, where RBBB was associated with increased risk (OR 4.75, 95% CI: 2.59–8.72; p<0.001) and use of the ACURATE neo was associated with decreased risk of PPI at 30 days (OR 0.63, 95% CI: 0.41-0.96; p=0.002) (Supplementary Table 5, Figure 2).There was no significant interaction of the effect of THV on PPI at 30 days across subgroups of pre-existing RBBB (pinteraction=0.447), history of atrial fibrillation (pinteraction=0.310), small aortic annuli (pinteraction=0.105), eccentric annuli (pinteraction=0.439) and aortic valve (pinteraction=0.145) and LVOT calcification (pinteraction=0.702) (Supplementary Figure 1).
There was no significant association between new PPI at 30 days and the clinical outcome at 1 year: neither for all-cause mortality (7 [7.2%] vs 42 [8.1%]; log-rank=0.775) (Figure 3A), nor symptomatic benefit in terms of NYHA Functional Class (Supplementary Figure 2A). While LV function significantly improved after TAVR, there was no difference at 30 days and 1 year between patients with or without PPI at 30 days (Supplementary Figure 3A).
Table 1. Baseline characteristics of the PPI 30 cohort according to the need for PPI at 30 days.| PPI 30 − n=541 |
PPI 30 + n=107 |
p-value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 83.2±4.3 | 82.7±3.8 | 0.258 |
| Female gender | 381 (70.4) | 76 (71.0) | 0.901 |
| Body mass index, kg/m2 | 27.1±5.0 | 27.6±5.2 | 0.297 |
| NYHA Class III or IV | 345 (63.8) | 62 (57.9) | 0.255 |
| EuroSCORE I, % | 11 [8-15] (n=512) | 11 [8-15] (n=101) | 0.369 |
| STS score, % | 4 [3-5] (n=531) |
4 [3-6] (n=105) |
0.139 |
| Diabetes mellitus | 144 (26.6) | 28 (16.3) | 0.923 |
| Hypercholesterolaemia | 270 (49.9) | 59 (55.1) | 0.323 |
| Arterial hypertension | 462 (85.4) | 89 (83.2) | 0.556 |
| Coronary artery disease | 207 (38.3) | 43 (40.2) | 0.709 |
| Previous myocardial infarction | 35 (6.5) | 11 (10.3) | 0.161 |
| Peripheral artery disease | 47 (8.7) | 11 (10.3) | 0.598 |
| COPD | 62 (11.5) | 10 (9.3) | 0.525 |
| ECG | |||
| History of atrial fibrillation | 167 (30.9) | 39 (36.4) | 0.257 |
| Bradycardia, beats/min | 97/529 (18.3) | 21/105 (20.0) | 0.689 |
| First degree atrioventricular block | 69/535 (12.9) | 15/107 (14.0) | 0.753 |
| Left bundle branch block | 48/530 (9.1) | 3/107 (2.8) | 0.030 |
| Right bundle branch block | 27/530 (5.1) | 25/107 (23.4) | <0.001 |
| QRS duration, ms | 100.02±23.19 (n=518) | 107.53±27.27 (n=103) | 0.004 |
| MSCT | |||
| Aortic annulus area, mm2 | 426.69±162.00 (n=521) | 422.70±54.00 (n=99) | 0.809 |
| Aortic annulus perimeter, mm | 73.7±4.8 (n=506) | 73.8±4.8 (n=99) | 0.801 |
| Moderate and severe aortic calcification | 375/533 (70.4) | 84/105 (80.0) | 0.044 |
| Moderate and severe LVOT calcification | 77/533 (14.4) | 22/104 (21.2) | 0.084 |
| Procedural characteristics | |||
| Conscious sedation | 469 (86.7) | 93 (86.9) | 0.950 |
| Predilatation | 326 (60.3) | 63 (58.9) | 0.790 |
| Post-dilatation | 222 (41.0) | 42 (39.3) | 0.732 |
| All data are mean±standard deviation, median [interquartile range] or absolute number (percentage). P-values are derived from chi-square or Fisher’s exact tests for categorical variables and Student’s t-tests or Wilcoxon rank-sum tests for continuous variables. In case of missing data, numbers of available measurements are given. COPD: chronic obstructive pulmonary disease; ECG: electrocardiogram; LVOT: left ventricular outflow tract; MSCT: multislice computed tomography; NYHA: New York Heart Association; PPI: permanent pacemaker implantation; STS: Society of Thoracic Surgeons | |||
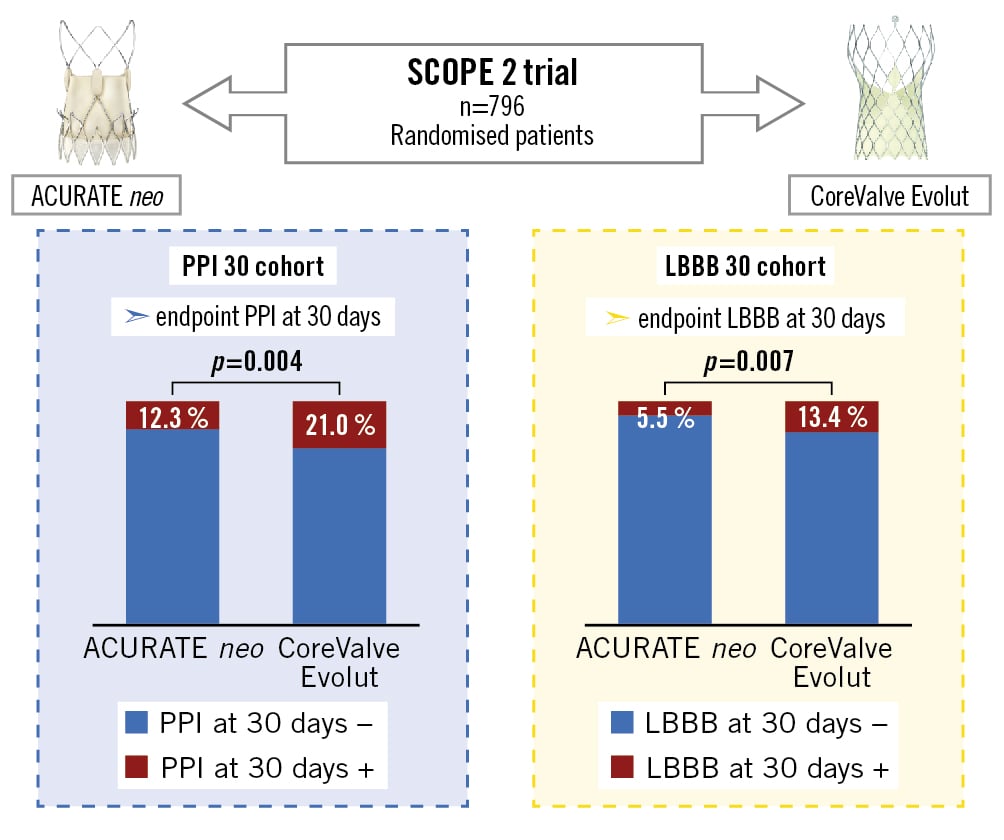
Central illustration. A SCOPE 2 subanalysis – randomised comparison of pacemaker and LBBB in the ACURATE neo and CoreValve Evolut. Comparison of the ACURATE neo and the CoreValve Evolut THV from the randomised SCOPE 2 trial, showing significantly lower rates of permanent pacemaker implantation and new-onset left bundle branch block at 30 days in ACURATE neo recipients. LBBB: left bundle branch block; PPI: permanent pacemaker implantation
ACURATE neo illustration provided courtesy of Boston Scientific. Copyright 2022 © Boston Scientific Corporation or its affiliates. All rights reserved. CoreValve Evolut R illustration provided courtesy of Medtronic GmbH.
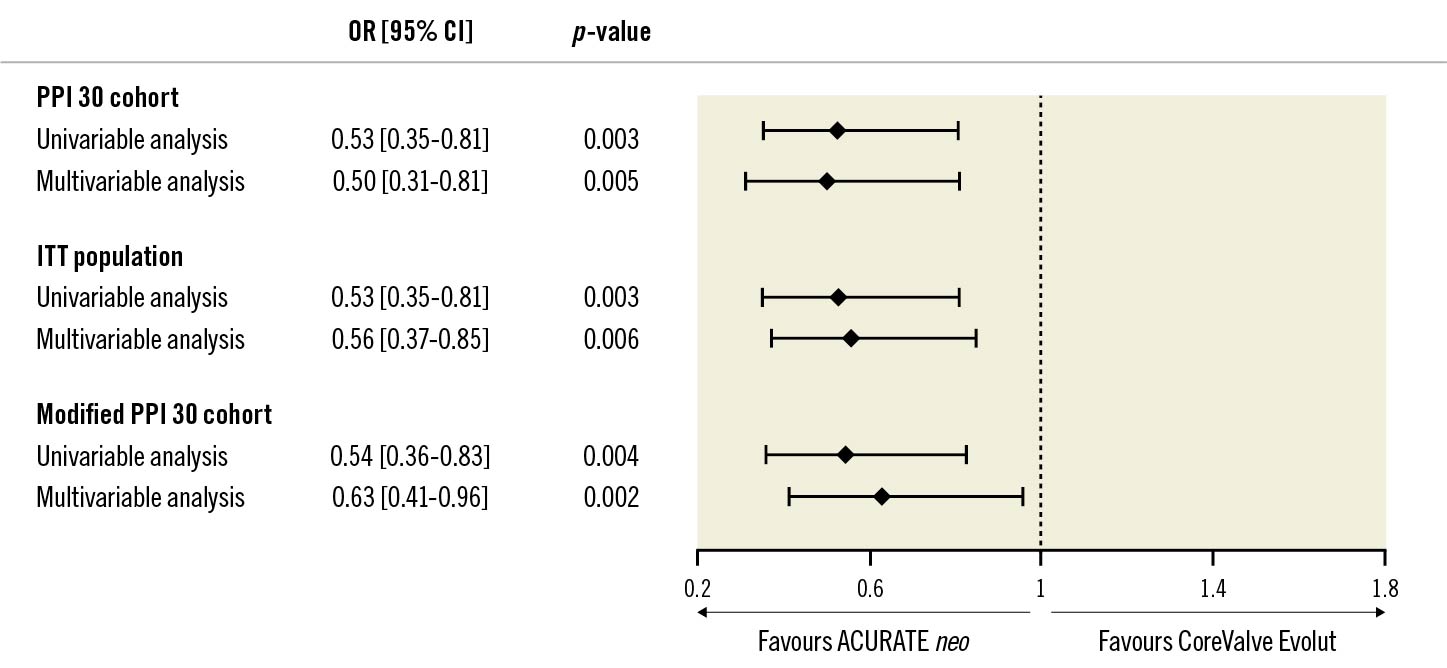
Figure 2. Risk of PPI at 30 days according to THV in the designated PPI 30 cohort, in the ITT population and in the modified PPI 30 cohort. Risk of PPI at 30 days according to THV. CI: confidence interval; ITT: intention-to-treat population; OR: odds ratio; PPI: permanent pacemaker implantation
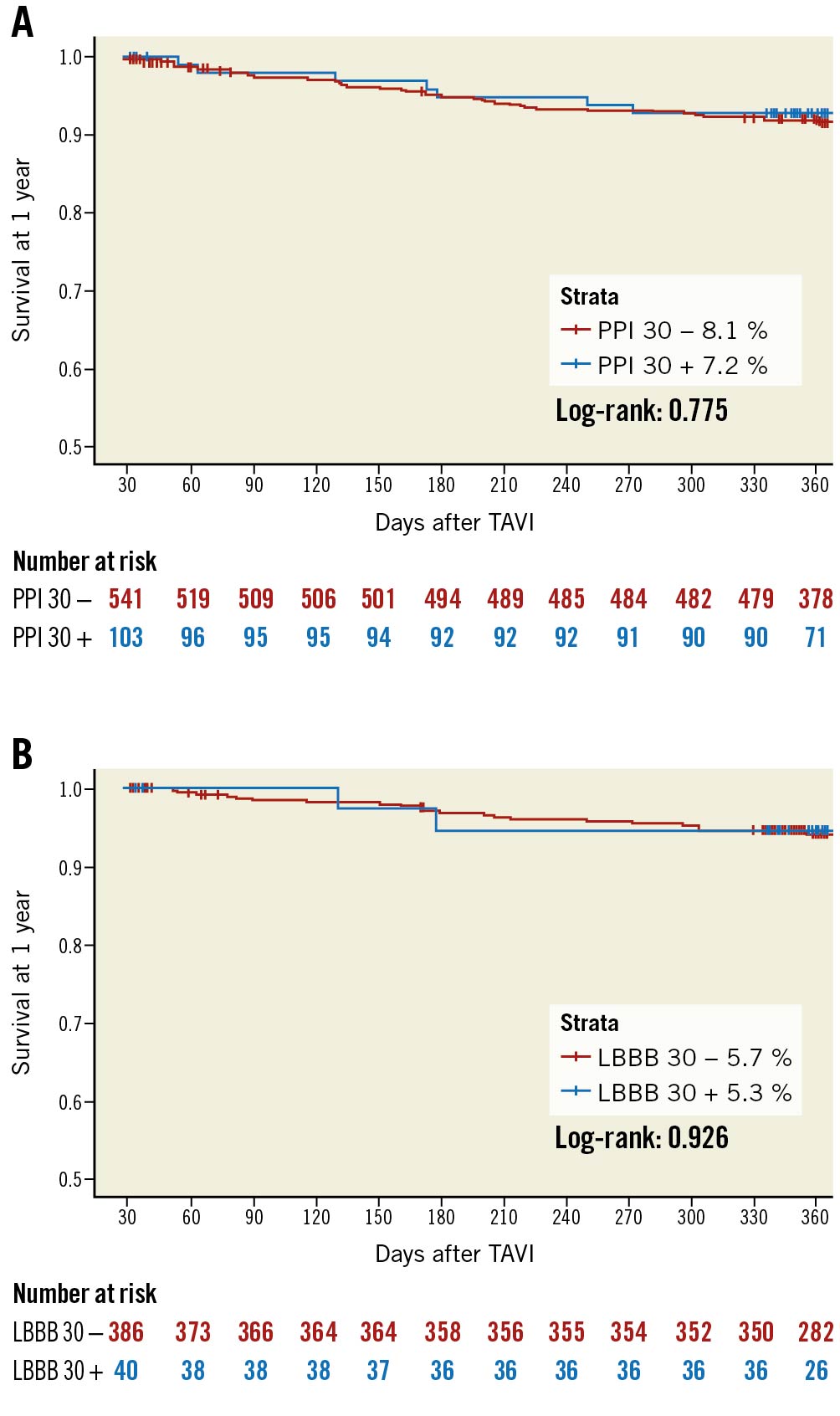
Figure 3. Survival according to new PPI at 30 days and new LBBB at 30 days. Kaplan-Meier survival curves for all-cause mortality stratified for new PPI at 30 days (A) and new LBBB at 30 days (B). LBBB: left bundle branch block; PPI: permanent pacemaker implantation; TAVR: transcatheter aortic valve replacement
New left bundle branch block – development and impact on outcome
Overall, 16.9% (72/426) of patients developed post-operative LBBB, which persisted in only 9.4% (40/426) at 30 days (Figure 4). The baseline characteristics of the LBBB 30 cohort according to the implanted THV are outlined in Supplementary Table 6 and showed no significant difference, except for AV block I, larger aortic annulus anatomies and higher rates of pre-and post-dilatation for the ACURATE neo compared to the CoreValve Evolut THV. Baseline and procedural characteristics according to new LBBB at 30 days are shown in Table 2: no significant differences were observed. In the univariable analysis, patients treated with the ACURATE neo showed significantly lower rates of new LBBB at 30 days compared to patients treated with the CoreValve Evolut THV (12 [5.5%] vs 28 [13.4%]; p=0.007) (Central illustration).
Patients who developed new LBBB at 30 days showed similar all-cause mortality (Figure 3B) and symptomatic benefit in terms of NYHA Functional Class at 1 year (Supplementary Figure 2B). LV function significantly improved after TAVR; however, LV function at 1 year was significantly lower in patients with new LBBB at 30 days compared to those without (65.7%±11.0 vs 69.1%±7.6; p=0.041) (Supplementary Figure 3B).
Table 2. Baseline characteristics of the new LBBB 30 cohort according to the presence of new LBBB at 30 days.| LBBB 30 − n=386 |
LBBB 30 + n=40 |
p-value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 82.7±4.1 | 84.0±4.3 | 0.077 |
| Female gender | 274 (71.0) | 26 (65.0) | 0.430 |
| Body mass index, kg/m2 | 27.2±5.0 | 26.6±4.7 | 0.471 |
| NYHA Class III or IV | 245 (63.5) | 25 (62.5) | 0.903 |
| EuroSCORE I, % | 11 [8-14] (n=360) | 10 [8-15] (n=37) | 0.500 |
| STS score, % | 3 [2-5] (n=376) |
4 [3-6] (n=40) |
0.067 |
| Diabetes mellitus | 98 (25.4) | 9 (22.5) | 0.688 |
| Hypercholesterolaemia | 186 (48.2) | 14 (35.0) | 0.112 |
| Arterial hypertension | 322 (83.4) | 30 (75.0) | 0.181 |
| Coronary artery disease | 152 (39.4) | 14 (35.0) | 0.589 |
| Previous myocardial infarction | 24 (6.2) | 2 (5.0) | 0.999 |
| Peripheral artery disease | 28 (7.9) | 3 (7.5) | 0.999 |
| COPD | 44 (11.4) | 5 (12.5) | 0.796 |
| ECG | |||
| History of atrial fibrillation | 116 (30.1) | 11 (27.5) | 0.737 |
| Bradycardia, beats/min | 70/383 (18.3) | 7/39 (17.9) | 0.960 |
| First degree atrioventricular block | 54 (14.0) | 8 (20.0) | 0.305 |
| QRS duration, ms | 97.04±21.01 (n=376) | 99.62±17.83 (n=39) | 0.460 |
| MSCT | |||
| Aortic annulus area, mm2 | 420.72±54.12 (n=372) | 411.86±56.44 (n=38) | 0.339 |
| Aortic annulus perimeter, mm | 73.7±4.8 (n=362) | 73.0±5.1 (n=37) | 0.370 |
| Moderate and severe aortic calcification | 286/384 (74.5) | 29/39 (74.4) | 0.987 |
| Moderate and severe LVOT calcification | 51/383 (13.3) | 3/39 (7.7) | 0.317 |
| Procedural characteristics | |||
| Conscious sedation | 330 (85.5) | 34 (85.0) | 0.933 |
| Predilatation | 246 (63.7) | 26 (65.0) | 0.874 |
| Post-dilatation | 160 (41.5) | 12 (30.0) | 0.160 |
| All data are mean±standard deviation, median [interquartile range] or absolute number (percentage). P-values are derived from chi-square or Fisher’s exact tests for categorical variables and Student’s t-tests or Wilcoxon rank-sum tests for continuous variables. In case of missing data, numbers of available measurements are given. COPD: chronic obstructive pulmonary disease; ECG: electrocardiogram; LBBB: left bundle branch block; LVOT: left ventricular outflow tract; MSCT: multislice computed tomography; NYHA: New York Heart Association; STS: Society of Thoracic Surgeons | |||
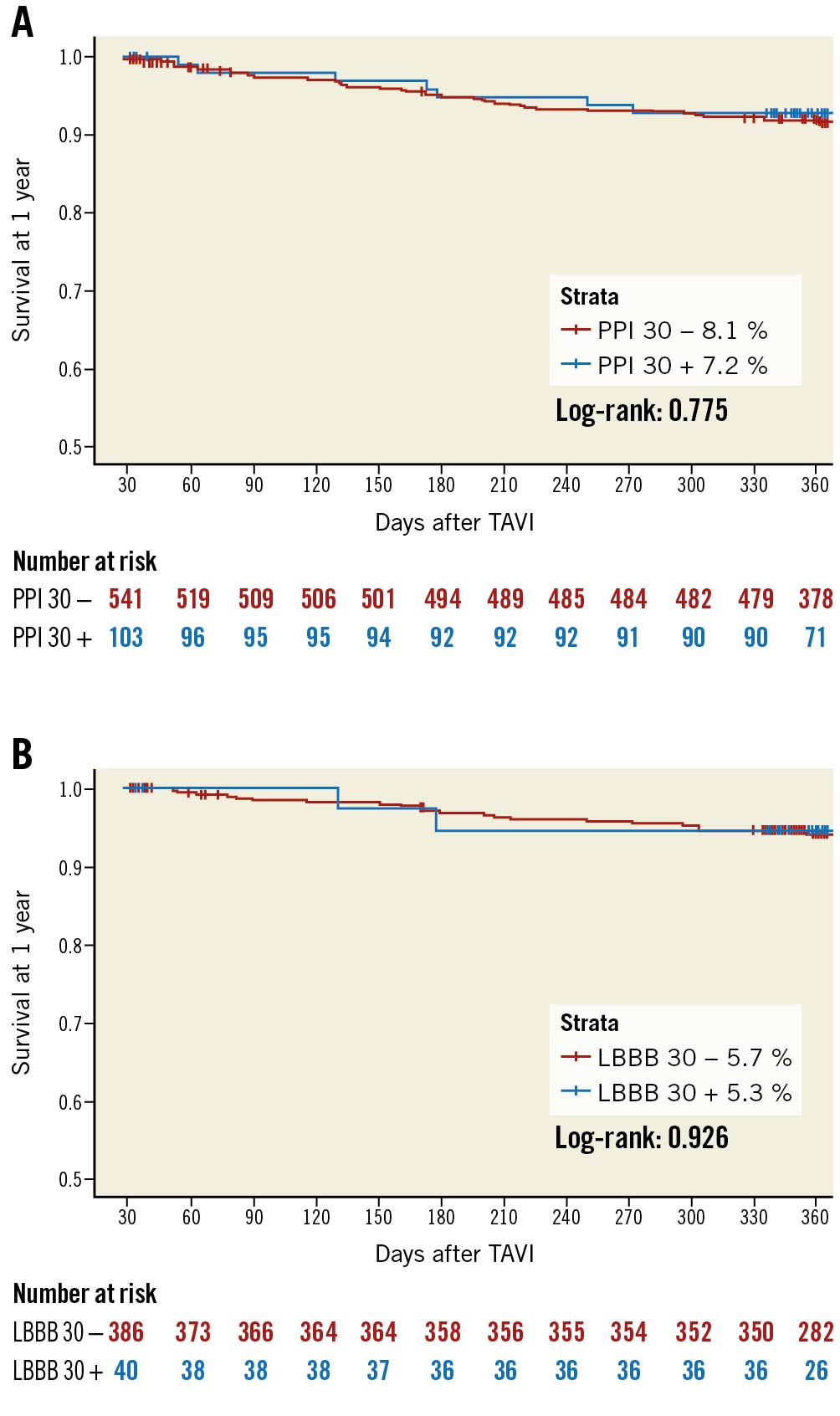
Figure 3. Survival according to new PPI at 30 days and new LBBB at 30 days. Kaplan-Meier survival curves for all-cause mortality stratified for new PPI at 30 days (A) and new LBBB at 30 days (B). LBBB: left bundle branch block; PPI: permanent pacemaker implantation; TAVR: transcatheter aortic valve replacement
Discussion
The results of this study can be summarised as follows: i) in an in-depth analysis of a randomised clinical trial, rates of new LBBB and new PPI at 30 days were significantly lower in patients treated with the ACURATE neo compared to the CoreValve Evolut THV; ii) pre-existing RBBB was associated with an increased, and the use of the ACURATE neo THV with a decreased, risk of PPI at 30 days; iii) at 1-year follow-up, there was no difference in clinical outcome regarding all-cause mortality in patients with or without new LBBB and new PPI at 30 days, respectively; iv) new LBBB at 30 days was associated with reduced LV function at 1 year.
New conduction abnormalities and the need for PPI remain the most frequent complications after TAVR, despite improvements in THV technology and adapted implantation strategies6. While early randomised comparisons between THV showed higher rates of conduction abnormalities and pacemaker rates with self-expanding THV compared to balloon-expandable THV18, more recent investigations showed favourable comparative results: use of the ACURATE neo THV led to comparable or even lower rates of PPI compared with balloon-expandable platforms, ranging from 2% to 10%111719. In contrast, conduction disturbances leading to pacemaker implantation remained high with early-generation CoreValve and Evolut R devices, ranging from 17.4% to 25.9%102021. However, subsequent CoreValve Evolut THV iterations showed improved outcome following technological adjustment and adoption of a refined implantation strategy: indeed, early results from an interim analysis of the Optimize PRO Study (ClinicalTrials.gov: NCT04091048) showed lower pacemaker rates of 8.8% at 30 days with the newest CoreValve Evolut PRO and PRO+ THV (Grubb K. An Optimized TAVR Care Pathway Using Evolut PRO and PRO+ Early Results from the Optimize PRO Study, SCAI 2021).
The SCOPE 2 trial, the only contemporary randomised clinical trial comparing the ACURATE neo and the CoreValve Evolut THV, was powered to detect a difference in the key secondary endpoint of new PPI at 30 days, and the ACURATE neo THV was found to be superior, with an absolute reduction of 7.5% in the intention-to-treat population compared to the CoreValve Evolut THV12. In the current substudy, designed to specifically analyse conduction abnormalities with these platforms, we confirmed original findings regarding PPI at 30 days, with lower rates for the ACURATE neo THV, as well as finding significantly lower rates of new LBBB at 30 days with the ACURATE neo THV.
Permanent pacemaker implantation – impact on outcome
Controversial data exist on the consequence of new PPI after TAVR: while some studies failed to show an adverse impact on mortality822, more recent analyses have consistently suggested impaired outcome with higher mortality and LV dysfunction923. In the current analysis we could not identify an association of new PPI with impaired outcome at 1 year. Possible explanations are an inadequately powered study population as well as insufficient follow-up. PPI induces ventricular dysfunction by right ventricular stimulation, which may occur after some time delay. Furthermore, no data were available on the stimulation rates in patients requiring new PPI, as right ventricular pacing >40% has been associated with a poor outcome24. Future analyses, set out to determine the need for initial PPI but also pacemaker dependency over time, are warranted to identify patients in which sustained right ventricular stimulation may lead to a worse outcome. Furthermore, the impact of the indication leading to PPI must be considered: while in the early TAVR experience, indication for PPI was liberal and generous, current practice has changed over the years and resulted in more restrictive indications for PPI after TAVR. The detrimental effects of PPI are related to foreign body-associated complications (i.e., infections) and long-term right ventricular pacing. A recent analysis comparing a liberal versus restrictive indication regimen for PPI showed that the restrictive cluster significantly reduced PPI rates after TAVR and led to a numerically, although not statistically, significant reduction in the composite of mortality and hospitalisation for heart failure at 3 years25.
With the perspective of extending TAVR to lower risk and younger patients, it is paramount to reduce post-procedural PPI rates, especially in light of the expected longer survival. Against this background, THV selection should aim for the lowest possible complication rates and a THV choice tailored to patients’ characteristics. In this analysis we found that the use of the ACURATE neo THV reduced the risk of new PPI at 30 days, promoting its use in patients at high risk for conduction abnormalities, such as those with pre-existing RBBB, one of the strongest PPI predictors in general2627. However, potential benefits should always be weighed against possible downsides. Compared to the CoreValve Evolut, the ACURATE neo THV showed higher rates of moderate-to-severe paravalvular regurgitation, which should be taken into consideration. As new iterations for both platforms, the ACURATE neo2 and the Evolut R PRO and PRO+, have recently become available, with refinements addressing previous shortcomings and adapted implantation techniques, new randomised clinical trials are warranted to corroborate the current findings. The DOUBLE-CHOICE randomised clinical trial (ClinicalTrials.gov: NCT05036018) is setting out to demonstrate non-inferiority of the ACURATE neo2 in comparison to the CoreValve Evolut PRO/PRO+ THV, and isolated local anaesthesia in comparison with local anaesthesia and conscious sedation, with respect to safety and efficacy in patients with severe symptomatic aortic stenosis undergoing TAVR.
New left bundle branch block – development and impact on outcome
Development of new LBBB is the most common conduction abnormality after TAVR with incidences ranging from 6% to 77%928. Multiple factors may influence these varying rates; first and foremost, the choice of THV: rates around 10-13% were described with the ACURATE neo THV, 12%-22% for the SAPIEN 3 THV and 19%-34% for the CoreValve Evolut THV, while the highest rates up to 77% were described with the mechanically expanding Lotus (Boston Scientific) THV15282930. However, another critical aspect to consider is the dynamic development of new LBBB over time: in the immediate post-procedural phase, new LBBB rates of 85% to 94% were described, with almost half of them regressing at discharge or at 30 days (range 44% to 65%). In line with these findings, in the current analysis we found that 55% of new LBBB at discharge resolved at 30 days. It remains paramount to identify patients with persistent LBBB, to better characterise the underlying conduction disturbances, and to identify which of these patients are at risk of developing secondary complications. Indeed, a recent study from the PARTNER II trial showed that new LBBB was associated with increased all-cause and cardiovascular mortality, rehospitalisation, new pacemaker implantation and worsened LV function at 2 years following the TAVR procedure7. Similarly, a meta-analysis, which included >42,000 patients, confirmed an increased risk of all-cause death and rehospitalisation for heart failure at 1 year in patients with new LBBB9. The pathophysiological mechanism underlying this association is multifactorial: mechanical dyssynchrony caused by LBBB may lead to LV dysfunction and subsequent heart failure. Furthermore, the risk of LBBB degenerating into complete AV block and resulting in sudden cardiac death should be considered9. Lastly, electrical dyssynchrony caused by LBBB may promote fatal ventricular arrhythmias7. In our study, we found no association between new persistent LBBB and mortality or rehospitalisation; however, we detected reduced LV function compared to patients without LBBB. Possibly, LBBB-induced dyssynchrony and subsequently reduced ejection fraction demonstrate a cause-and-effect relationship, where longer follow-up is warranted to detect the impact on mortality.
Limitations
The findings of this study need to be interpreted in light of several limitations. Firstly, while the SCOPE 2 trial was powered to detect differences in pacemaker implantations, it was not powered to show differences with regard to individual clinical endpoints, such as new LBBB. Secondly, the participating centres had different levels of experience in the implantation of the ACURATE neo THV. In some countries the THV only became available with the participation in this study, possibly influencing the results. Furthermore, a limited clinical follow-up of 1 year and incomplete electrocardiographic and echocardiographic data during follow-up may preclude the identification of significant long-term outcomes, especially in light of the current analysis regarding the impact of new LBBB and PPI on mortality and LV function. Detailed information on THV delivery and implantation, in terms of implantation depth, recapturing and repositioning, which may have influenced the occurrence of LBBB and PPI, were not systematically collected. Information on ventricular pacing during follow-up was not available, thus precluding further analyses in this regard. The SCOPE 2 trial was not powered for the performed subgroup analyses; therefore, the results have to be considered carefully as hypothesis-generating statements.
Conclusions
In conclusion, in this in-depth analysis of the randomised SCOPE 2 clinical trial, we found that new conduction abnormalities and new PPI are significantly lower when using the ACURATE neo compared to the CoreValve Evolut THV. Right bundle branch block (increased risk) and use of the ACURATE neo (reduced risk) were the only independent predictors of PPI. Although no effect on mortality was determined for new PPI at 30 days, the development of new LBBB at 30 days was associated with reduced ejection fraction at 1 year.
Impact on daily practice
The development of post-operative new left bundle branch block (LBBB) and need for new permanent pacemaker implantations (PPI) persist as concerning complications after transcatheter aortic valve replacement with a possible adverse prognostic impact. In this comparison from a randomised clinical trial, we performed a dedicated analysis of the incidence and impact of new LBBB and PPI using 2 new-generation self-expanding devices, the ACURATE neo and the CoreValve Evolut. Both, LBBB and PPI rates were significantly lower in ACURATE neo compared to CoreValve Evolut recipients. Furthermore, use of the ACURATE neo was associated with a decreased risk of PPI. Besides reduced left ventricular function at 1 year in patients with new LBBB, no impact on mortality was found for patients with LBBB or PPI at 1 year.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Funding
This trial was sponsored by CERIC (Center for European Research Initiatives in CardiovascularMedicine) with support from a dedicated research grant from Symetis SA (Ecublens, Switzerland).
Conflict of interest statement
C. Tamburino has received speaker fees from Medtronic. S. Bleiziffer has received speaker fees from Boston Scientific, Edwards Lifesciences, Abbott, and Medtronic. M. Cunnington has received speaker fees from Medtronic, Boston Scientific, and Abbott Vascular; and support for attending educational meetings and travel from Medtronic, Boston Scientific, and Edwards Lifesciences.A. Wolf is a proctor for Boston Scientific, Edwards Lifesciences, and Medtronic. M. Barbanti has received consultant fees from Edwards Lifesciences, Medtronic and Boston Scientific. P. Pagnotta is a proctor for Boston Scientific, Cardia, and Gore Medical. F. Bedogni has received personal fees from Abbott Vascular, Boston Scientific, Medtronic, Meril Life Sciences, and Terumo. E. Van Belle has received personal fees from Philips Volcano. M. Vasa-Nicotera is a proctor for Boston Scientific and Medtronic. A. Chieffo has received speaker fees from Abiomed, Abbott Vascular, Biosensors, Cardinal Health, and Magenta; and consultant fees from Abiomed, Abbott Vascular, Biosensors, Cardinal Health, and Magenta. K. Bogaerts has received consultant fees from the Cardiovascular European Research Center. C. Hengstenberg is a proctor for Edwards Lifesciences and Boston Scientific; reports institutional grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic; and has received speaker and consultant fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. D. Capodanno has received speaker's fee from Medtronic (institutional), Daiichi Sankyo and Sanofi. M. Joner reports institutional research grants from Cardiac Dimensions, Infraredx, and Edwards Lifesciences; has received consulting fees from Boston Scientific, Biotronik, Cardiac Dimensions, and Shockwave; speaker fees from Abbott, AstraZeneca, Biotronik, Shockwave, Boston Scientific, Edwards Lifesciences, and OrbusNeich; he reports participation on the Data Safety Monitoring or Advisory Board of Biotronik, and Shockwave; and personal fees from AstraZeneca, Biotronik, Boston Scientific, Edwards Lifesciences, OrbusNeich, and ReCor. H. Thiele reports being President elect of the German Cardiac Society; ESC Guideline Task Force Chair NSTE-ACS 2020; and ESC Guideline Task Force Chair ACS 2023. C. Pellegrini reports receiving a personal research grant from Else Kröner Fresenius Stiftung. J. Cockburn is proctor for Acurate neo and reports receiving speaker fees for talks at EuroPCR. P. Garot received speaker fees from Abbott, Biosensors, Boston Scientific, Edwards Lifesciences, and General Electric HealthCare; and is a shareholder and Medical Director of CERC. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

