Abstract
Aims: Transcatheter aortic valve implantation (TAVI) is frequently complicated by new left bundle branch block (LBBB). We investigated the development and persistence of LBBB during follow-up and its clinical consequences.
Methods and results: ECGs at baseline, within 24 hours, before discharge and at 12 months after TAVI were assessed in 476 patients without pre-existing LBBB and/or pacemaker before or after TAVI. TAVI-induced new LBBB was categorised based on the timing of the occurrence (within 24 hours [acute], after 24 hours but before discharge [subacute], and after discharge [late]), in addition to persistence (transient or persistent). A total of 175 patients (36.8%) developed new LBBB of which 85.7% occurred within 24 hours after TAVI, 12.0% before and 2.3% after hospital discharge, and was persistent in 111 patients (63.4%). Implantation of the Medtronic CoreValve System (MCS) more frequently led to new LBBB than the balloon-expandable Edwards SAPIEN valve (ES) (53.8% versus 21.7%) with less recovery during follow-up (39.0% versus 9.5%). Late new LBBB was only seen in four patients (0.8%). During a median follow-up of 915 (578-1,234) days, persistent LBBB was associated with a significant increase in mortality as compared to no LBBB and temporary LBBB combined (hazard ratio 1.49, 95% confidence interval, 1.10-2.03; p=0.01).
Conclusions: TAVI-induced new LBBB occurs in almost 40% of patients, almost all before hospital discharge. It occurs three times more frequently after MCS than after ES valve implantation and has a twofold lower tendency to resolve during follow-up. Persistent LBBB is associated with a higher mortality.
Introduction
Since the first successful implantation in 20021, transcatheter aortic valve implantation (TAVI) has become an accepted and evidence-based alternative to surgical aortic valve replacement in selected patients with aortic valve stenosis2,3. Despite its clinical benefits, periprocedural conduction disorders, in particular new left bundle branch block (new LBBB), frequently occur after TAVI4-6. New LBBB affects left ventricular function, increases the risk for postoperative permanent pacemaker implantation and has been associated with increased mortality4,5,7,8.
New LBBB occurs more often after implantation of the self-expanding Medtronic CoreValve System (MCS; Medtronic, Minneapolis, MN, USA), with a reported frequency of 30-60%, than after the balloon-expandable Edwards SAPIEN valve (ES; Edwards Lifesciences, Irvine, CA, USA), with a reported frequency of 6-12%6,9-13.
There are, however, scant detailed electrocardiographic data assessing the changes of QRS duration and morphology not only shortly after TAVI but also during follow-up. Recovery of TAVI-induced new LBBB may occur but is less frequent after MCS than ES valve implantation. Also, little is known about the development of intraventricular conduction disorders after hospital discharge5,14-16.
This was the subject of the present study in which a series of 476 patients who underwent TAVI with the MCS or ES device without pre-existing LBBB, permanent pacemaker (PPM) or postprocedural PPM implantation were subjected to a detailed and prospective electrocardiographic assessment.
Methods
PATIENT POPULATION
The patient population consisted of 701 patients who underwent TAVI between January 2006 and July 2011 with the Medtronic CoreValve System (MCS) (n=339) or the balloon-expandable Edwards SAPIEN valve (ES) (n=350) in any of the following institutions: Quebec Heart & Lung Institute, Quebec, Canada (n=212; ES: n=206), Erasmus Medical Center, Rotterdam, The Netherlands (n=202; MCS: n=200), Catharina Hospital, Eindhoven, The Netherlands (n=173; MCS: n=139; ES: n=30), Maastricht University Medical Center, Maastricht, The Netherlands (n=114; ES: n=114). In 12 patients the procedure was aborted without implantation of any valve.
For the purpose of the study, only patients with a minimum follow-up of one year after TAVI were eligible. Also, patients with pre-existing LBBB and/or permanent pacemaker (PPM) before TAVI were excluded from analysis, as well as patients who did not undergo valve implantation (aborted procedure). Patients who received a new PPM within 30 days after TAVI were also excluded, since it precludes accurate assessment of eventual LBBB or other conduction disorders. Therefore, the study population consisted of 484 patients (Figure 1), of whom six patients (1.2%) died during or shortly after the procedure, resulting in the absence of any postprocedural electrocardiogram (ECG). For another two patients (0.4%) there were no ECGs available after the implantation.
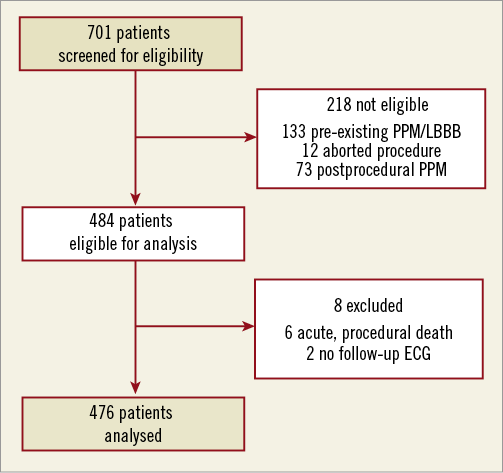
Figure 1. Study population. PPM: permanent pacemaker; LBBB: left bundle branch block
All clinical and procedural data were prospectively collected and entered into a dedicated central database. If necessary, additional information was collected by analysis of medical records. The use of anonymous clinical, procedural and follow-up data for research was in accordance with the policies of the institutions.
OBJECTIVES & DATA COLLECTION
The primary objective was to assess the changes in intraventricular conduction by comparing the 12-lead ECGs at baseline, within 24 hours, before discharge and 12 months after TAVI. ECG tracings were stored digitally in either the portable document (PDF) or Joint Photographic Experts Group (JPEG) format, depending on availability per patient and centre. All tracings were analysed by an experienced cardiologist (PH) to record heart rhythm, PR interval, QRS duration, QRS morphology and QRS axis in exact degrees. Digital files were zoomed to 800% to measure intervals and duration. The presence of first, second or third degree atrioventricular block, right bundle branch block (RBBB), LBBB, left anterior hemiblock (LAHB) and left posterior hemiblock (LPHB) were recorded according to the established criteria17. Accordingly, LBBB was defined as a V1-negative QRS complex of ≥0.12 seconds in duration with absent Q-waves and a notched or slurred R in leads I, aVL, V5 and/or V6. An LAHB was defined as a QRS duration ≥0.10 seconds with a frontal plane QRS axis between –45 and –90 degrees in the presence of a qR in leads I and aVL. In the presence of RBBB, LAHB was defined as a frontal plane QRS axis between –45 and –90 degrees. Finally, a significant change in QRS duration was defined as an absolute change of more than 30 milliseconds (msec), based on reported interobserver variability of measured QRS duration18. Examples of the ECG interpretation are shown in Figure 2.
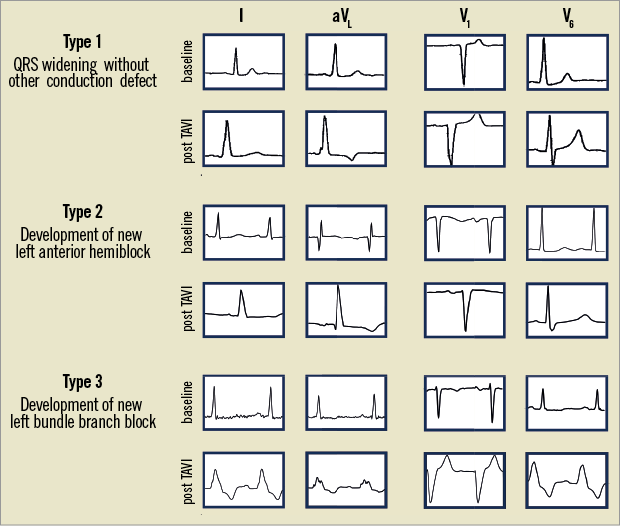
Figure 2. Examples of changes in QRS duration and/or morphology. Illustration of different patterns of change in QRS duration and/or morphology after TAVI. Type 1 indicates QRS widening >120 msec without distinct conduction defect and types 2 and 3 are an example of new LAHB and new LBBB, respectively. Although there is a significant widening (>30 msec) of the QRS complex in type 1, this should not be considered a new LBBB.
The occurrence of and recovery from LBBB were studied by comparing ECGs between the different time points. Distinction was made between acute LBBB (onset within 24 hours after TAVI), subacute LBBB (onset after 24 hours but before discharge) and late LBBB (onset after discharge). In addition, persistent LBBB was defined as any LBBB which was present 12 months after TAVI, and transient LBBB as new LBBB resolved within 12 months. In patients who died before one-year follow-up (n=50; 10.5%) and in those without an ECG at one year after TAVI (n=34; 7.1%), the last available ECG was used for classification of transient or persistent LBBB.
The secondary objective was to compare mortality among patients with temporary, persistent and no LBBB. Mortality was checked by contacting the Civil Registry in the Netherlands which continuously records all deaths and causes of death for all Dutch citizens and inhabitants of the Netherlands. For the Canadian study population, mortality was checked by contacting the referring physician or general practitioner.
STATISTICAL ANALYSIS
Categorical variables are presented as numbers and proportions. For continuous variables, normality of distribution was assessed with the Kolmogorov-Smirnov test. Normal and skewed continuous variables are presented as means with standard deviation (SD) and medians with interquartile range (IQR), respectively.
Baseline variables between patients without a new LBBB, and patients with transient LBBB or persistent LBBB after TAVI were compared using repeated measures analysis of variance (ANOVA) in case of a continuous measurement. Binary logistic regression analysis was used to compare categorical variables. Where applicable, variables were compared using the unpaired t-test or Mann-Whitney U test for normal and skewed continuous variables, respectively. Categorical variables were compared using the Pearson chi-square test. Survival was estimated using the Kaplan-Meier method. Log-rank testing was used to compare differences in survival among patients without, with transient and with persistent LBBB. Survival was also compared between patients with persistent and patients without persistent LBBB (i.e., patients with transient or no LBBB) using both log-rank testing and Cox regression analysis. In addition, Kaplan-Meier estimates of survival were constructed for patients who received a PPM after TAVI.
A two-sided p-value less than 0.05 was considered to be statistically significant. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS), version 20 (IBM SPSS, Chicago, IL, USA).
Results
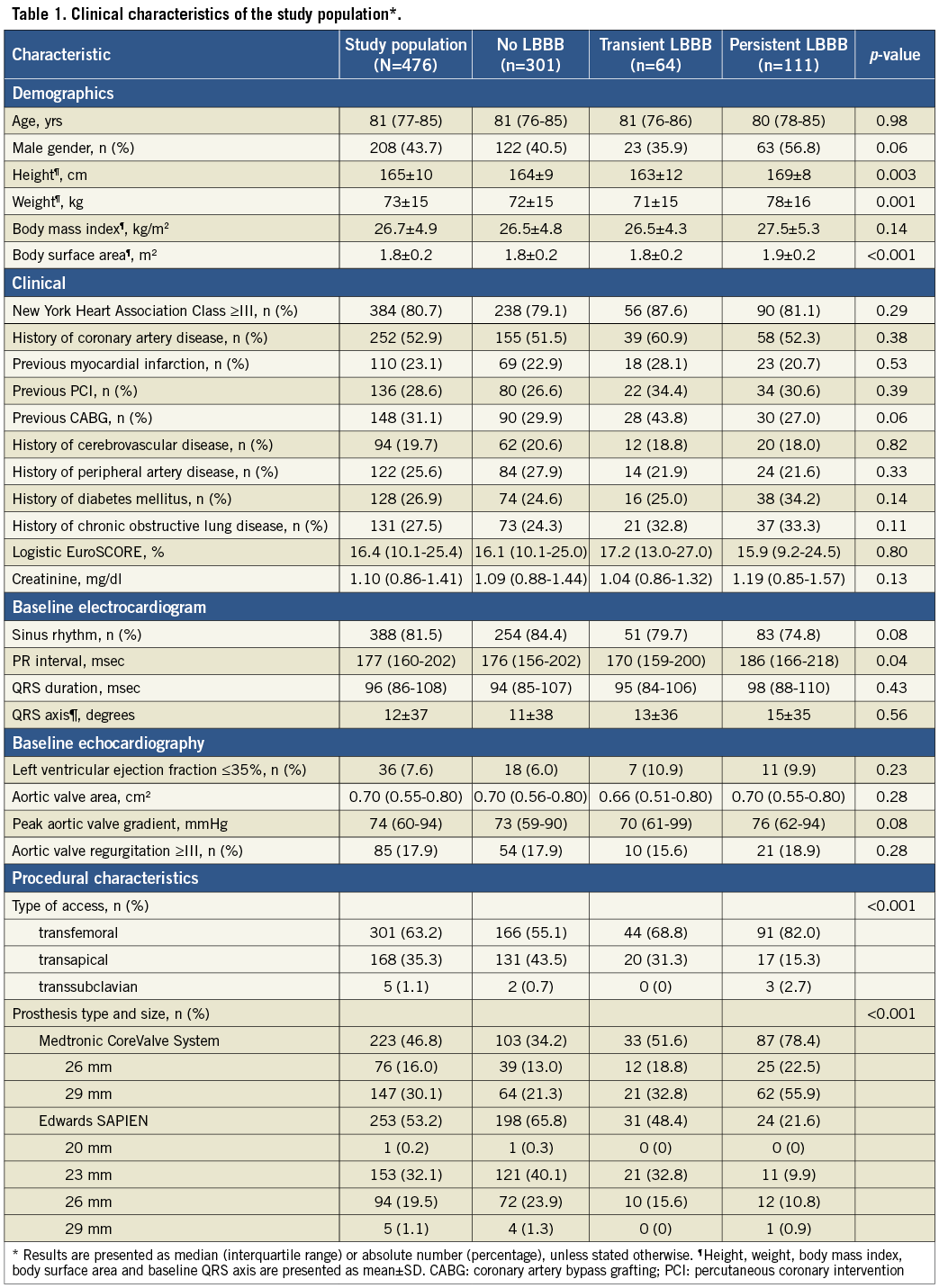
Baseline characteristics and procedural details of the study population of 476 patients eligible for analysis (Figure 1) and of those with a transient and persistent LBBB (Figure 3) are shown in Table 1. Overall, there was an almost even distribution of both devices (MCS in 223 patients or 46.8%; ES in 253 patients or 53.2%). The majority of patients (301 or 63.2%) underwent transfemoral TAVI and 168 (35.3%) underwent transapical TAVI.
There were 175 patients (36.8%) who developed a new LBBB: this occurred within 24 hours after TAVI (acute LBBB) in 150 patients (31.5%), >24 hours but before hospital discharge (subacute LBBB) in 21 (4.4%), and after discharge (late LBBB) in four patients (0.8%) (Figure 3). At 12 months, TAVI-induced new LBBB was persistent in 111 out of 175 patients (63.4%) and transient in 64 (36.6%).
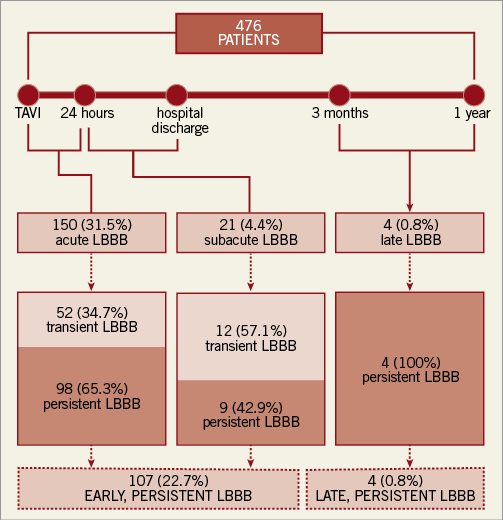
Figure 3. Frequency, timing and persistence of TAVI-induced new LBBB. LBBB: left bundle branch block
ECG details are shown in Table 2. A new LAHB was the second most frequent ventricular conduction disorder and occurred in 17.2% (n=76) out of the 442 patients without LAHB at baseline, and was persistent in 57 (75%). A new RBBB occurred in 12 patients (2.7%) without baseline RBBB (n=446). Most conduction disorders occurred before discharge. A new LBBB, LAHB and RBBB occurred during follow-up in four, seven and one patient(s), respectively.
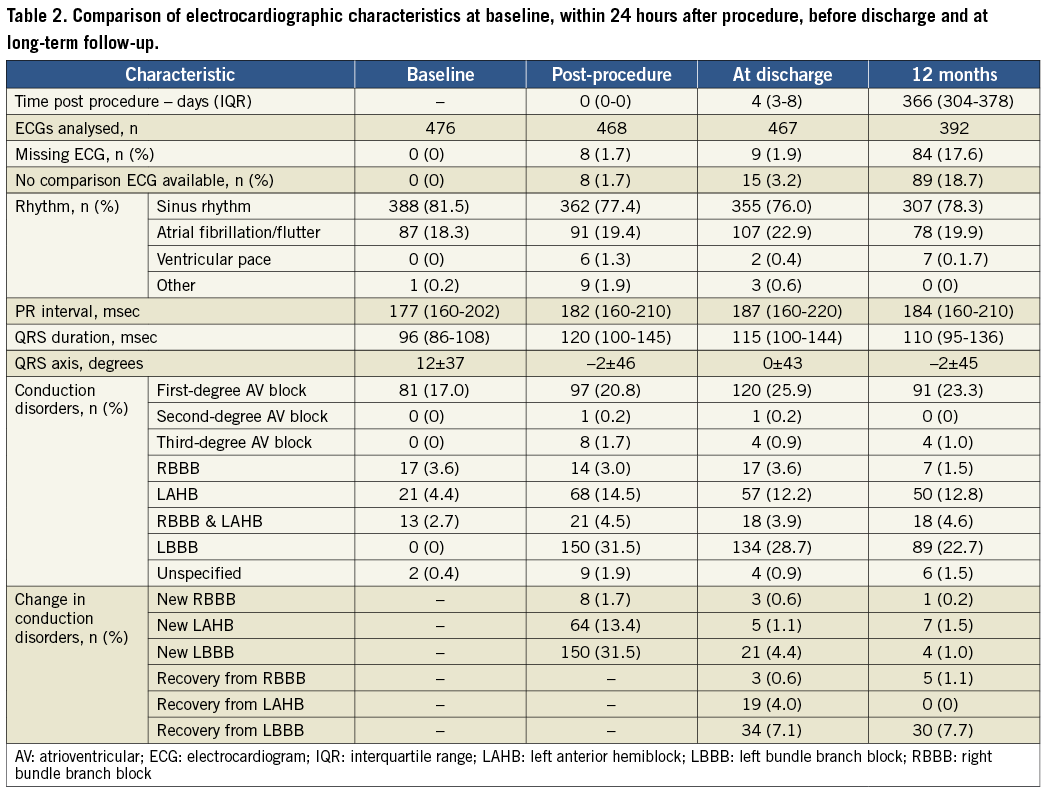
By univariate analysis, a new LBBB occurred more frequently after MCS than after ES valve implantation, and was also more often persistent (53.8% and 39.0% for MCS versus 21.7% and 9.5% for ES, respectively; p<0.001) (Table 1, Table 3). As the transfemoral route is associated with MCS implantation, this access route was also more frequent in patients who developed new LBBB. However, a new LAHB was more frequent after ES valve implantation (27.5% versus 5.3%; p<0.001) and was also more often persistent (20.3% versus 4.4%; p<0.001).
OUTCOME (MORTALITY AT FOLLOW-UP)
Median follow-up was 898 (592-1,183), 944 (691-1,321) and 914 (268-1,333) days in patients without, with temporary and with persistent LBBB, respectively (p=0.08). Mortality at one year was 17.3% (n=52), 6.2% (n=4) and 27.0% (n=30) in patients without LBBB, with temporary LBBB and with persistent LBBB, respectively, and was 38.2% (n=115), 31.2% (n=20) and 53.2% (n=59) at total follow-up (Figure 4A). When comparing patients with persistent LBBB and patients without persistent LBBB (i.e., combining patients without LBBB and patients with temporary LBBB), mortality at total follow-up was 37.0% (n=135) and 53.2% (n=59) for patients without and with persistent LBBB, respectively (Figure 4B). Using the univariate regression model, the hazard of mortality was 1.49 (95% confidence interval, 1.10-2.03; p=0.01). In total, 73 patients received a PPM within 30 days after TAVI, in whom the mortality at total follow-up was 47.9% (n=35) (Figure 4B). The indication of PPM after TAVI was total atrioventricular block in the majority of patients (75.3%; n=55), and 19.2% (n=14) had LBBB in the postprocedural period before PPM implantation.
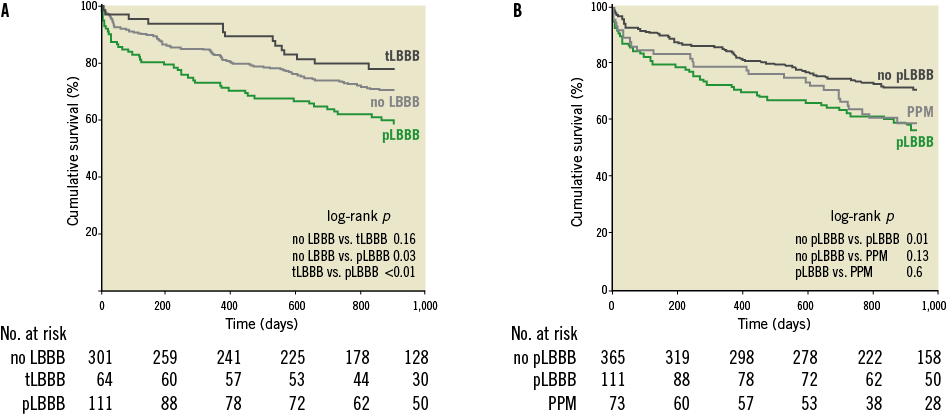
Figure 4. Kaplan-Meier survival estimates of patients without, with temporary and with persistent LBBB. A) compares survival of patients without, with temporary and with persistent LBBB. B) compares survival between patients with persistent and without persistent LBBB. The survival curve of patients who received a permanent pacemaker (PPM) within 30 days is also shown in panel B. Comparison was made using the log-rank test. “No LBBB” denotes patients without left bundle branch block (LBBB) induced by transcatheter aortic valve implantation (TAVI), “tLBBB” patients with temporary LBBB, and “pLBBB” patients with persistent LBBB.
Discussion
This study demonstrates that approximately 40% of patients develop a new LBBB after TAVI, most of which persists at follow-up. A new LBBB occurs 2.5 times more often after MCS than after ES valve implantation and is also associated with less recovery. Persistent LBBB is associated with a worse prognosis (i.e., higher mortality during follow-up). These findings contribute to a better insight into the occurrence, persistence and consequence of TAVI-induced LBBB.
Acknowledging the absence of direct comparisons between different valves, a consistently higher frequency of new LBBB has been reported after MCS (29-65%)11,19 than after ES valve implantation (4-18%)20,21. Given the differences in design, mode of implantation and action, the difference between the valves is plausible but does not explain the variation in LBBB frequency of each valve separately6. This variation may in part be due to intrinsic features of observational research22 and variations and difficulties in the application of diagnostic criteria of LBBB as illustrated in Figure 2. We also believe that, in addition to the morphologic ECG criteria, the timing of occurrence (within 24 hours, before and after hospital discharge) and recovery of new LBBB should be considered, as demonstrated by Urena et al and by the present study5. The present study does not allow us to elucidate as to whether the prognosis in case of a persistent LBBB differs between MCS and ES implantation. A difference in mortality is conceivable, given the lesser recovery of the conduction abnormality after MCS implantation, but remains to be proven. The sample size of the present study, however, does not allow a valid analysis of an eventual different prognostic effect between the valves.
At variance with observations in a smaller series (in which a lower frequency and degree of persistence of new LAHB was reported), we found that new LAHB occurred more often and persisted more after ES valve than after MCS valve implantation21,23. The difference in new LAHB between the valves may be explained by the fact that a much higher number of patients have a new (complete) LBBB after MCS valve implantation. While new LBBB is known to be associated with a decrease in left ventricular function, a higher risk of complete AV block and impaired survival, the prognostic effects of a new LAHB after TAVI remain to be established24,25.
In concordance with a previous observation revealing a higher mortality in patients with an LBBB after TAVI at discharge4, we found a higher mortality during follow-up in patients with a persistent new LBBB. These results are supported by a recent study, showing that mortality after TAVI increases with increasing QRS duration26. In conflict with these studies, however, a recent Italian multicentre registry showed no difference in mortality between patients without and with new LBBB on the ECG before hospital discharge27. This discrepancy between studies may be explained by differences in the baseline risk of the study population, the application of diagnostic ECG criteria and differences in the degree of persistence of new LBBB. Therefore, prognostic factors other than LBBB may have played a more dominant role in the outcome of these patients. Furthermore, it is conceivable that an adverse prognostic effect is only seen in patients with a persistent LBBB. We found that up to 35% of LBBB recovers at follow-up. A difference in the degree of persistence between the present study and the Italian study population may also explain the discrepancy. Registries comparing both the MCS and the ES prostheses in large patient populations (U.K. TAVI, FRANCE 2, PRAGMATIC)28-30 did not find a difference in one-year mortality. The rate of postprocedural PPM implantation, however, was approximately three times higher for the MCS valve. These patients are protected from bradyarrhythmias thus influencing outcome.
The nature of the present study does not allow us to establish the cause of death or reason why patients with a persistent LBBB after TAVI suffer from an increased mortality. The increased risk of death in these patients may be explained by dyssynchrony-induced heart failure which may have negative effects in elderly and hypertrophic hearts in particular.
TAVI-induced LBBB has been reported to be associated with a decrease in LV ejection fraction (LVEF) similar to the adverse effects of LBBB in patients or individuals with and without cardiovascular disease5,7,8,31. Of note, a recent study reported a substantial increase in hospitalisation of patients with a moderate increase in QRS duration indicating that decreased cardiac performance was the cause of clinical deterioration26. The prognostic effects of LBBB are further underscored by observations in a wide spectrum of patients with and without cardiovascular disease and the fact that after cardiac resynchronisation therapy a reduction of 53% in both mortality and heart failure is seen in LBBB patients32,33. Another potential cause of death may be progression to complete heart block, as has been demonstrated in patients with LBBB after surgical aortic valve implantation34. Survival of patients with new PPM is intermediary between survival of patients with and without persistent LBBB. This may be explained by the fact that these patients are protected from bradyarrhythmic death, but not from dyssynchrony-induced heart failure.
Limitations
The main limitation of the study is its observational nature. Therefore, it does not provide a full insight into the pathophysiology of the observations. For instance, the depth of implantation was not included, something which is known to play an important role in LBBB development5,19,20. This, in addition to the number of patients, precluded a multivariate analysis for assessment of predictors of both transient and persistent new LBBB. Echocardiographic data were not systematically available, which precluded the assessment of the influence of LBBB on left ventricular function. Although the ECGs were analysed by an experienced cardiologist (PH) using established criteria of conduction disorders, independent core lab analysis was not performed. The median follow-up of the present study was approximately 2.5 years. The causes of mortality are manifold. Therefore, analysis of mortality in larger populations with longer follow-up may help to increase our understanding of the prognostic effects of new persistent LBBB after TAVI.
Conclusions
TAVI-induced new LBBB occurs in almost 40% of patients, most of which occurs before hospital discharge. It occurs 2.5 times more frequently after MCS than after ES valve implantation and has a twofold lower tendency to resolve. Late new LBBB occurs rarely. Persistent LBBB is associated with a higher mortality.
Conflict of interest statement
P. Houthuizen received a speakers fee from Edwards Lifesciences; L. Van Garsse is a proctor for Edwards Lifesciences; J. Rodés-Cabau is a consultant for Edwards Lifesciences and St. Jude Medical; F. Prinzen received research grants from Medtronic, EBR Systems, Philips Healthcare, Proteus and Enopace. The other authors have no conflicts of interest to declare.

