Abstract
Background: The ACURATE neo2 (NEO2) and Evolut PRO/PRO+ (PRO) bioprostheses are new-generation self-expanding valves developed for transcatheter aortic valve replacement (TAVR).
Aims: We sought to compare the performance of the ACURATE neo2 and Evolut PRO/PRO+ devices.
Methods: The NEOPRO-2 registry retrospectively included patients who underwent TAVR for severe aortic stenosis with either the NEO2 or PRO devices between August 2017 and December 2021 at 20 centres. In-hospital and 30-day Valve Academic Research Consortium (VARC)-3 defined outcomes were evaluated. Propensity score (PS) matching and binary logistic regression were performed to adjust the treatment effect for PS quintiles. A subgroup analysis assessed the impact of aortic valve calcification.
Results: A total of 2,175 patients (NEO2: n=763; PRO: n=1,412) were included. The mean age was 82±6.2 years and the mean Society of Thoracic Surgeons score was 4.2%. Periprocedural complications were low, and both groups achieved high rates of technical success (93.1% vs 94.1%; p=0.361) and predischarge intended valve performance (96.0% vs 94.1%; p=0.056), both in the unmatched and matched analysis (452 pairs). Device success at 30 days was comparable (84.3% vs 83.6%; p=0.688), regardless of aortic valve calcification severity (p>0.05 for interaction). A suggestion for higher VARC-3 early safety in the NEO2 group was mainly driven by reduced rates of new permanent pacemaker implantation (7.7% vs 15.6%; p<0.001).
Conclusions: This retrospective analysis reports a similar short-term performance of the ACURATE neo2 platform compared with the new-generation Evolut PRO/PRO+ devices. Randomised studies are needed to confirm our exploratory findings.
Introduction
Transcatheter aortic valve replacement (TAVR) is an established treatment in patients with severe aortic stenosis (AS)123456. Transcatheter heart valve (THV) design has evolved to meet the high standards required for the application of TAVR in a younger and healthier population. Therefore, head-to-head comparisons of new-generation THV are useful to tailor device selection.
The ACURATE neo2 (NEO2) bioprosthesis (Boston Scientific) is a new-generation self-expanding THV which has been commercially available in Europe since September 2020. Its precursor, the ACURATE neo (NEO) device, despite encouraging results in observational studies, including the NEOPRO registry, did not meet non-inferiority to the Evolut R/PRO devices in the Safety and Efficacy Comparison of Two TAVI Systems in a Prospective Randomized Evaluation 2 (SCOPE II) trial for the primary endpoint of all-cause death or stroke at 1 year. In addition, moderate or severe paravalvular aortic regurgitation (AR) was a major concern78910111213. Therefore, the NEO2 design focused on improved sealing, with a 60% larger skirt compared to the first-generation NEO, to minimise paravalvular AR. Data from the ACURATE neo2 Conformité Européenne (CE) Mark Study were promising: procedural success was high with a low rate of moderate or severe paravalvular leak (PVL) at 1 year (2.5%)14. A recent analysis from the NEOPRO and NEOPRO-2 registries confirmed a significant reduction in predischarge moderate or severe PVL with the ACURATE neo2 device compared with its precursor (2% vs 5%; p<0.001)15. Since the new-generation Evolut PRO and PRO+ (PRO) bioprostheses (Medtronic) achieved high standards in terms of safety and efficacy, they represent the benchmark for self-expanding devices1617. Therefore, the aim of our study was to compare TAVR with the latest-generation ACURATE neo2 and Evolut PRO and PRO+ bioprostheses in order to understand whether technology iteration impacts on device performance and short-term outcomes.
Methods
Study population
NEOPRO-2 (A Multicenter Comparison of ACURATE NEO2 Versus Evolut PRO/PRO+ Transcatheter Heart Valves 2) was an international, observational, retrospective registry that included consecutive patients who underwent transfemoral TAVR for severe symptomatic aortic stenosis with either NEO2 or PRO devices between August 2017 and December 2021 at 20 centres15. A total of 2,175 patients were included in the registry: 763 patients (35.1%) treated with NEO2; 1,412 patients (64.9%) treated with Evolut PRO/PRO+ (n=158/1,412 [11.2%] with PRO+). The number of patients included from each participating centre is detailed in Supplementary Table 1. The treatment periods were September 2020 to December 2021 and August 2017 to October 2021 for the NEO2 and PRO groups, respectively.
Local multidisciplinary Heart Teams evaluated all cases and confirmed eligibility for transfemoral TAVR for symptomatic, severe stenosis of the native aortic valve (AV). All patients provided written informed consent for the procedure and subsequent data collection per local practice for retrospective data. Preprocedural screening was performed by means of clinical assessment (patient demographics, symptoms, comorbidities, laboratory examinations, and risk evaluation), echocardiography, and multidetector computed tomography (MDCT). AV and left ventricular outflow tract calcifications were classified and graded using a semiquantitative scoring system, as previously described18. The selection of prosthesis type and size was at the discretion of the treating physician at each centre.
Device description
The NEO2 device preserves several characteristics of its precursor, the ACURATE neo bioprosthesis, including a self-expanding nitinol frame with relatively low radial force, porcine pericardial leaflets in a supra-annular position, and self-aligning stabilisation arches with open-cell geometry19. In addition, it presents 2 new features: a 60% larger pericardial inner and outer skirt, to enhance sealing, and a radiopaque marker, for more precise positioning. Three sizes are available: small, medium, and large, which correspond to annular diameters up to 23, 25, and 27 mm, respectively. It is implanted using a delivery system inserted through a 14 Fr expandable sheath (iSleeve; Boston Scientific), as previously described20.
The self-expanding supra-annular Evolut PRO bioprosthesis shares similar properties with the second-generation Evolut R THV, including an identical frame and inner tissue21. The principal design modification is the presence of an external pericardial wrap on the 23, 26, and 29 mm valves to enhance sealing with a 16 Fr delivery profile. The Evolut PRO+ device was developed to introduce the additional sealing skirt to the 34 mm valve and to reduce the dimension of the delivery sheath profile (14 Fr).
Study endpoints
The primary endpoint of the study was 30-day device success, defined according to Valve Academic Research Consortium-3 (VARC-3) criteria22. Secondary endpoints of interest included additional VARC-3-defined composite outcomes: technical success, predischarge intended performance of the valve, 30-day early safety, and the single components of these endpoints. Echocardiographic outcomes were evaluated predischarge and at 30 days; AR severity was assessed according to VARC-3 criteria and classified as none/trace, mild, moderate, and severe.
Statistical analysis
Continuous variables are presented as mean±standard deviation and were compared using the unpaired Student’s t-test. Categorical variables are presented as numbers and percentages and were compared using the chi-square test.
Propensity score (PS) matching was used to adjust for differences in baseline characteristics, as previously described23. A PS was calculated for each patient to estimate the propensity toward belonging to a specific treatment group (NEO2 vs PRO). This was done by means of a non-parsimonious multivariate logistic regression including the following covariates: age, sex, body mass index, chronic obstructive pulmonary disease, estimated glomerular filtration rate, prior percutaneous coronary intervention, peripheral vascular disease, atrial fibrillation/flutter, New York Heart Association (NYHA) Functional Class III-IV, left ventricular ejection fraction (LVEF), European System for Cardiac Operative Risk Evaluation (EuroSCORE) II, moderate-to-heavy AV calcification, and AV annulus perimeter. The C-statistic for the PS model was 0.65, indicating good discrimination. A 1-to-1 nearest neighbour matching algorithm without replacement (calliper 0.05) was performed to identify PS-matched pairs. The pseudo-R2 value was 0.0423 (p<0.0001) before matching and very low (0.005; p=0.953) after matching, thus confirming the adequate balancing of covariate distribution between the matched groups24.
Prespecified primary and secondary endpoints were compared between the NEO2 and PRO groups in the overall and PS-matched cohorts. Binary logistic regression was performed to adjust the treatment effect for the PS quintiles in the overall cohort; results are presented as adjusted odds ratio (aOR) with 95% confidence interval (CI). In addition, we conducted a subgroup analysis of 30-day outcomes in patients grouped according to the severity of AV calcifications: none-mild (n=368 [23.7%]), moderate (n=709 [43.6%]), and heavy (n=550 [33.8%]).
All reported p-values are 2-sided, and a p-value <0.05 was considered as indicating statistical significance. All statistical analyses were performed using Stata version 13.0 (StataCorp).
Results
Baseline characteristics
A total of 2,175 patients who underwent TAVR with either NEO2 (n=763) or PRO/PRO+ (n=1,412) THV from August 2017 to December 2021 were included. Baseline characteristics are summarised in Table 1. The mean age was 81.7±6.2 years, and the mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 4.2±2.8%. Patients treated with PRO/PRO+ devices were more frequently males, had more frequently a history of prior cardiac surgery and more than mild mitral regurgitation, together with a lower LVEF and worse NYHA Class. Whereas patients in the NEO2 group more frequently reported a history of peripheral vascular disease, previous percutaneous coronary intervention, and atrial fibrillation/flutter. Similar annular perimeter was observed at MDCT between the groups, whereas moderate-to-heavy AV calcification was more frequent in the NEO2 group.
Table 1. Baseline patient characteristics.
| Overall (n=2,175) |
ACURATE neo2 (n=763) | Evolut PRO/PRO+ (n=1,412) | p-value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years) | 81.7±6.2 | 81.7±5.9 | 81.7±6.4 | 0.908 |
| Male sex | 809/2,175 (37.2) | 251/763 (32.9) | 558/1,412 (39.5) | 0.002 |
| BMI | 27.1±5.2 | 27.2±5.0 | 27.1±5.2 | 0.927 |
| COPD | 320/2,171 (14.7) | 125/762 (16.4) | 195/1,409 (13.8) | 0.108 |
| Diabetes mellitus | 656/2,169 (30.2) | 220/763 (28.8) | 436/1,406 (31.0) | 0.292 |
| Arterial hypertension | 1,851/2,172 (85.2) | 648/763 (84.9) | 1,203/1,409 (85.4) | 0.777 |
| eGFR (ml/min/m2) | 60.8±27.0 | 63.6±28.6 | 59.2±25.9 | <0.001 |
| Dialysis | 45/2,175 (2.1) | 11/763 (1.4) | 34/1,412 (2.4) | 0.131 |
| Prior PCI | 498/2,172 (22.9) | 213/763 (27.9) | 285/1,409 (20.2) | <0.001 |
| Prior cardiac surgery | 180/2,171 (8.3) | 53/763 (6.9) | 127/1,408 (9.0) | 0.005 |
| Prior CABG | 140/2,172 (6.5) | 47/763 (6.2) | 93/1,409 (6.6) | 0.690 |
| Peripheral vascular disease | 275/2,171 (12.7) | 115/763 (15.1) | 160/1,408 (11.4) | 0.013 |
| Prior stroke | 203/2,171 (9.4) | 83/763 (10.9) | 120/1,408 (8.5) | 0.072 |
| Atrial fibrillation/flutter | 579/2,166 (26.7) | 240/755 (31.8) | 339/1,411 (24.0) | <0.001 |
| PM or ICD | 191/2,173 (8.8) | 61/763 (8.0) | 130/1,409 (9.2) | 0.329 |
| NYHA Class III or IV | 1,293/2,164 (59.8) | 417/761 (54.8) | 876/1,403 (62.4) | 0.001 |
| STS-PROM (%) | 4.2±2.8 | 4.2±3.1 | 4.2±2.7 | 0.602 |
| EuroSCORE II (%) | 4.5±4.2 | 4.3±3.9 | 4.7±4.4 | 0.060 |
| Echocardiographic data | ||||
| AVA (cm2) | 0.70±0.20 | 0.71±0.26 | 0.70±0.17 | 0.533 |
| LVEF (%) | 56.9±10.4 | 57.9±10.0 | 56.3±10.5 | <0.001 |
| Moderate to severe MR | 525/2,043 (25.7) | 160/705 (22.7) | 365/1,338 (27.3) | 0.024 |
| Moderate to severe TR | 251/1,762 (14.3) | 86/686 (12.5) | 165/1,076 (15.3) | 0.101 |
| Severe pulmonary hypertension* | 143/1,859 (7.7) | 56/659 (8.5) | 87/1,200 (7.3) | 0.334 |
| MDCT data | ||||
| Annular perimeter (mm) | 73.5±5.8 | 73.7±5.1 | 73.4±6.1 | 0.211 |
| Moderate to heavy AV calcification | 1,259/1,627 (77.4) | 444/629 (70.6) | 815/998 (81.7) | <0.001 |
| Any LVOT calcification | 524/1,066 (49.2) | 175/328 (53.4) | 349/738 (47.3) | 0.068 |
| Moderate to severe LVOT calcification | 220/1,066 (20.6) | 61/328 (18.6) | 159/738 (21.5) | 0.272 |
| Values are mean±SD or n/N (%). *Systolic pulmonary artery pressure on echocardiography >70 mmHg. AV: aortic valve; AVA: aortic valve area; BMI: body mass index; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; EuroSCORE: European System for Cardiac Operative Risk Evaluation; ICD: implantable cardioverter-defibrillator; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; MDCT: multidetector computed tomography; MR: mitral regurgitation; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; PM: pacemaker; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TR: tricuspid regurgitation | ||||
Procedural characteristics
As depicted in Table 2, most patients underwent TAVR under conscious sedation, with a significantly lower rate in the PRO group (86.1% vs 94.6%; p<0.001). Predilatation was more frequent in the NEO2 group (85.9% vs 44.3%; p<0.001), whereas post-dilatation rates were comparable between groups. Both groups achieved high rates of VARC-3 technical success (93.1% vs 94.1%; p=0.361) with no significant differences in periprocedural complications, except for higher vascular access complications in the PRO group (12.2% vs 8.5%; p=0.002), driven by minor vascular complications. Overall, procedural mortality occurred in 8 patients (0.4%); annular rupture was reported in 4 patients (0.2%) and all 4 cases underwent predilatation or post-dilatation.
Table 2. Procedural characteristics and predischarge echocardiographic outcomes.
| Overall (n=2,175) |
ACURATE neo2 (n=763) | Evolut PRO/PRO+ (n=1,412) | p-value | ||
|---|---|---|---|---|---|
| Procedural characteristics | |||||
| Conscious sedation | 1,938/2,175 (89.1) | 722/763 (94.6) | 1,216/1,412 (86.1) | <0.001 | |
| Transfemoral TAVR | 2,175/2,175 (100) | 763/763 (100) | 1,412/1,412 (100) | 1.000 | |
| Valve size | 23 mm (or S size) | – | 185/763 (24.2) | 49/1,233 (4.0) | – |
| 25 mm (or M size) | – | 327/763 (42.8) | – | ||
| 26 mm | – | – | 468/1,233 (38.0) | ||
| 27 mm (or L size) | – | 252/763 (33.0) | – | ||
| 29 mm | – | – | 682/1,233 (55.3) | ||
| 34 mm | – | – | 34/1,233 (2.7) | ||
| Predilatation | 1,278/2,169 (58.9) | 655/763 (85.9) | 623/1,406 (44.3) | <0.001 | |
| Post-dilatation | 587/2,039 (28.8) | 233/761 (30.6) | 354/1,278 (27.7) | 0.159 | |
| Procedural death | 8/2,175 (0.4) | 2/763 (0.3) | 6/1,412 (0.4) | 0.549 | |
| Second THV implanted | 19/2,172 (0.9) | 6/762 (0.8) | 13/1,410 (0.9) | 0.748 | |
| Valve embolisation | 23/2,172 (1.1) | 8/762 (1.1) | 15/1,410 (1.1) | 0.976 | |
| Annular rupture | 4/2,175 (0.2) | 1/763 (0.1) | 3/1,412 (0.2) | 0.672 | |
| Pericardial tamponade | 19/2,175 (0.9) | 7/763 (0.9) | 12/1,412 (0.9) | 0.872 | |
| Aortic dissection | 1/2,175 (0.1) | 0/763 (0.0) | 1/1,412 (0.1) | 0.462 | |
| Coronary occlusion | 10/2,175 (0.5) | 2/763 (0.3) | 8/1,412 (0.6) | 0.317 | |
| Conversion to cardiac surgery | 8/2,175 (0.4) | 3/763 (0.4) | 5/1,412 (0.4) | 0.886 | |
| Vascular access complications | Minor | 158/2,175 (7.3) | 35/763 (4.6) | 123/1,412 (8.7) | 0.002 |
| Major | 80/2,175 (3.7) | 30/763 (3.9) | 50/1,412 (3.5) | ||
| VARC-3 defined technical success | 2,038/2,175 (93.7) | 710/763 (93.1) | 1,328/1,412 (94.1) | 0.361 | |
| Echocardiographic outcomes | |||||
| Total aortic regurgitation | None/trace | 1,245/2,144 (58.1) | 424/752 (56.4) | 821/1,392 (59.0) | 0.003 |
| Mild | 826/2,144 (38.5) | 315/752 (41.9) | 511/1,392 (36.7) | ||
| Moderate | 69/2,144 (3.2) | 13/752 (1.7) | 56/1,392 (4.0) | ||
| Severe | 4/2,144 (0.2) | 0/752 (0.0) | 4/1,392 (0.3) | ||
| Moderate or severe paravalvular aortic regurgitation | 70/2,144 (3.3) | 13/752 (1.7) | 57/1,392 (4.1) | 0.003 | |
| Mean gradient ≥20 mmHg | 35/2,103 (1.7) | 14/747 (1.9) | 21/1,356 (1.6) | 0.577 | |
| Mean gradient (mmHg) | 8.2±4.1 | 9.1±4.2 | 7.7±4.0 | <0.001 | |
| Max gradient (mmHg) | 15.2±7.4 | 16.7±7.8 | 14.6±7.2 | <0.001 | |
| Aortic EOA (cm2) | 1.86±0.51 | 1.79±0.46 | 1.94±0.54 | <0.001 | |
| VARC-3 defined intended performance of the valve | 2,001/2,112 (94.7) | 719/749 (96.0) | 1,282/1,363 (94.1) | 0.056 | |
| Values are n/N (%) or mean±SD. EOA: effective orifice area; TAVR; transcatheter aortic valve replacement; THV: transcatheter heart valve; VARC-3: Valve Academic Research Consortium-3 | |||||
Early echocardiographic outcomes
Predischarge echocardiographic findings after TAVR are reported in Table 2. Both devices achieved high rates of VARC-3-defined intended performance of the valve (96.0% vs 94.1%; p=0.056). As depicted in Figure 1, AR after TAVR was mainly caused by PVL; moderate or severe AR was lower after a NEO2 implantation (1.7% vs 4.3%; p=0.003). The mean AV gradient was slightly higher in the NEO2 cohort (9.1±4.2 mmHg vs 7.7±4.0 mmHg; p<0.001); nevertheless, the proportion of patients with a mean AV gradient ≥20 mmHg was similar between the groups.
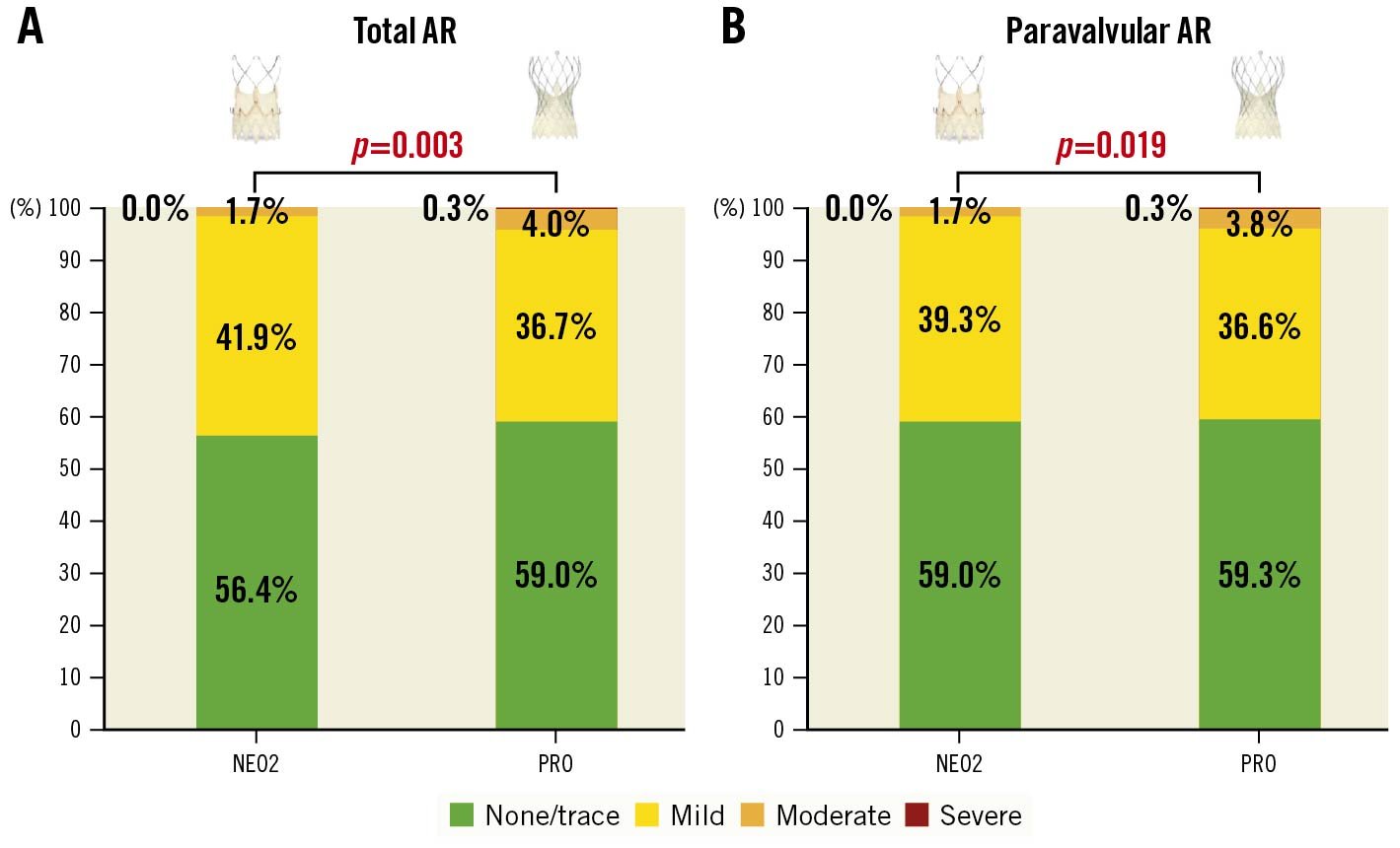
Figure 1. Aortic regurgitation after transcatheter aortic valve replacement. Predischarge AR after TAVR. Comparison of predischarge total (A) and paravalvular (B) AR after implantation of NEO2 and PRO devices.
AR: aortic regurgitation; NEO2: ACURATE neo2; PRO: Evolut PRO/PRO+; TAVR: transcatheter aortic valve replacement
VARC-3-defined outcomes at 30-day follow-up
Information on 30-day survival status was available for 2,158 of 2,175 patients (99.2%), with 53 deaths reported (overall all-cause mortality rate 2.5%) and 18 patients lost to follow-up.
As reported in Table 3, 30-day all-cause death and stroke rates (including disabling and non-disabling strokes) were acceptable and similar between both groups. Despite higher rates of hospitalisation for cardiovascular reasons and myocardial infarction in the NEO2 group, no differences in cardiovascular mortality emerged. New permanent pacemaker implantation (PPI) at 30 days was more frequently needed in the PRO cohort (15.6% vs 7.7%; p<0.001). In addition, lower rates of any bleeding (11.5% vs 17.3%; p=0.001) and vascular complications (5.7% vs 12.7%; p<0.001) were observed in the NEO2 group, driven by a reduction in Type 1 bleeding and minor vascular complications, respectively. Nevertheless, patients in the NEO2 group more frequently developed stage 3 or 4 acute kidney injury (AKI) (2.5% vs 1.2%; p=0.020). Echocardiographic data at 30 days strengthened predischarge results (Table 3). All other 30-day clinical outcomes were numerically low and similar in both groups.
VARC-3 device success (primary endpoint) was 83.8% in the overall cohort and similar in the NEO2 and PRO groups (84.3% vs 83.6%; p=0.688). The VARC-3 early safety composite endpoint at 30 days was more frequently achieved after TAVR with the NEO2 device (78.7% vs 71.3%; p<0.001).
After adjustment for PS quintiles, the implanted valve did not have a significant impact on 30-day VARC-3 device success in the overall cohort (aOR 0.77, 95% CI: 0.55-1.07; p=0.121). As shown in Supplementary Table 2, after adjustment for PS quintiles, NEO2 implantation was associated with a higher risk of cardiovascular hospitalisations and stage 3 or 4 AKI and with a lower risk of any vascular complications and new PPI. A similar risk in VARC-3 early safety (aOR 1.29, 95% CI: 0.97-1.71; p=0.082) and VARC-3 intended performance of the valve (aOR 1.10, 95% CI: 0.59-2.07; p=0.766) was observed between the NEO2 and PRO groups.
After 1-to-1 PS matching (for the variables summarised in “Methods”), a total of 452 pairs were obtained from the overall cohort (Supplementary Table 3). The PS-matched comparison substantially confirmed the results on procedural characteristics, periprocedural complications, and the predischarge haemodynamic outcomes that emerged in the overall population. Whereas there was no significant difference in terms of moderate or severe PVL between the matched NEO2 and PRO groups (Supplementary Table 4), VARC-3 intended performance of the valve, VARC-3 device success, and VARC-3 early safety were similar between the matched NEO2 and PRO groups (Supplementary Table 5). The lower rate of new PPI in the NEO2 group was also confirmed after PS matching.
Table 3. 30-day outcomes.
| Overall (n=2,175) |
ACURATE neo2 (n=763) | Evolut PRO/PRO+ (n=1,412) | p-value | ||
|---|---|---|---|---|---|
| Clinical outcomes | |||||
| All-cause mortality | 53/2,158 (2.5) | 22/760 (2.9) | 31/1,398 (2.2) | 0.332 | |
| Cardiovascular mortality | 43/2,158 (2.0) | 17/760 (2.2) | 26/1,398 (1.9) | 0.549 | |
| Stroke | 60/2,102 (2.9) | 20/709 (2.8) | 40/1,393 (2.9) | 0.597 | |
| Cardiac hospitalisation* | 61/2,103 (2.9) | 30/709 (4.2) | 31/1,394 (2.2) | 0.010 | |
| MI | 2/2,103 (0.1) | 2/709 (0.3) | 0/1,394 (0.0) | 0.047 | |
| VARC-3 bleeding | Type 1 | 181/2,103 (8.6) | 39/709 (5.5) | 142/1,394 (10.2) | 0.001 |
| Type 2 | 94/2,103 (4.5) | 23/709 (3.2) | 71/1,394 (5.1) | ||
| Type 3 | 45/2,103 (2.1) | 18/709 (2.5) | 27/1,394 (1.9) | ||
| Type 4 | 3/2,103 (0.1) | 2/709 (0.3) | 1/1,394 (0.1) | ||
| Vascular complications | Minor | 140/2,103 (6.7) | 18/709 (2.5) | 122/1,394 (8.7) | <0.001 |
| Major | 78/2,103 (3.7) | 23/709 (3.2) | 55/1,394 (4.0) | ||
| Access non-vascular complications | 0/2,103 (0.0) | 0/709 (0.0) | 0/1,394 (0.0) | 1.000 | |
| Permanent PM implantation** | 249/1,929 (12.9) | 51/663 (7.7) | 198/1,266 (15.6) | <0.001 | |
| Valve dysfunction requiring repeat intervention (BAV, TAVR, SAVR) | 6/2,117 (0.3) | 2/723 (0.3) | 4/1,394 (0.3) | 0.686 | |
| Valve embolisation/migration | 6/2,117 (0.3) | 3/723 (0.4) | 3/1,394 (0.2) | 0.412 | |
| Endocarditis | 3/2,117 (0.1) | 0/723 (0.0) | 3/1,394 (0.2) | 0.212 | |
| THV thrombosis | 3/2,117 (0.1) | 2/723 (0.3) | 1/1,394 (0.1) | 0.235 | |
| Intervention for cardiac structural complication | 6/2,117 (0.3) | 4/723 (0.5) | 2/1,394 (0.2) | 0.093 | |
| AKI stage 3 or 4 | 34/2,117 (1.6) | 18/723 (2.5) | 16/1,394 (1.2) | 0.020 | |
| NYHA Class III or IV | 41/1,078 (3.8) | 11/326 (3.4) | 30/752 (4.0) | 0.628 | |
| Valve performance and VARC-3 defined outcomes | |||||
| Moderate or severe total aortic regurgitation | 72/2,135 (3.4) | 17/750 (2.3) | 55/1,385 (4.0) | 0.037 | |
| Moderate or severe paravalvular aortic regurgitation | 72/2,135 (3.4) | 17/750 (2.3) | 55/1,385 (4.0) | 0.037 | |
| Mean gradient >20 mmHg | 36/2,094 (1.7) | 16/745 (2.2) | 20/1,349 (1.5) | 0.262 | |
| VARC-3 device success | 1,748/2,085 (83.8) | 606/719 (84.3) | 1,142/1,366 (83.6) | 0.688 | |
| VARC-3 early safety | 1,547/2,095 (73.8) | 566/719 (78.7) | 981/1,376 (71.3) | <0.001 | |
| VARC-3 intended performance of the valve | 2,001/2,107 (95.0) | 714/747 (95.6) | 1,287/1,360 (94.6) | 0.340 | |
| Values are n/N (%). *Including hospitalisation for valve-related symptoms or other cardiovascular reason **Excluding patients with pacemaker at baseline. AKI: acute kidney injury; BAV: balloon aortic valvuloplasty; MI: myocardial infarction; NYHA: New York Heart Association; PM: pacemaker; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve; VARC-3: Valve Academic Research Consortium-3 | |||||
Subgroup analysis on AV calcification
An exploratory subgroup analysis was performed to evaluate the main 30-day outcomes across different degrees of AV calcification in the overall cohort (Supplementary Table 6). Trends towards lower VARC-3 early safety in the PRO group among patients with heavy AV calcification and a lower rate of moderate-severe PVL in the NEO2 group among patients with none-mild AV calcification were observed. However, no significant interaction between the type of implanted THV and AV calcification severity was observed for all evaluated endpoints (all p-values for interaction>0.05). Of note, the higher rate of new PPI in the PRO group was confirmed in all AV calcification subgroups.
Discussion
The NEOPRO-2 registry compared short-term VARC-3-defined outcomes in 2,175 patients undergoing transfemoral TAVR with the new-generation ACURATE neo2 and Evolut PRO/PRO+ bioprostheses in a contemporary, real-world, multicentre setting. The main findings of our study are as follows: 1) despite baseline heterogeneity in patient characteristics, periprocedural complications were numerically low, and both groups achieved high rates of VARC-3-defined technical success and predischarge intended performance of the valve; 2) 30-day VARC-3 device success was 83.8% in the overall cohort, similar between the NEO2 and PRO devices; 3) despite a different rate of short-term complications, a possible advantage for the NEO2 group in terms of VARC-3 early safety in the entire population did not reach statistical significance after adjustment for PS quintiles and PS matching; 4) TAVR with the NEO2 THV resulted in lower rates of PPI compared to the PRO/PRO+ devices with no differences per grade of AV calcification.
In-hospital outcomes
The patient population included in the registry was heterogeneous at baseline, suggesting a potential selection bias toward and operator preference for the use of the Evolut platform in more challenging anatomies with a higher burden of AV calcification. Nevertheless, both devices achieved high rates of VARC-3-defined technical success both in the unmatched (NEO2 93.1%, PRO 94.1%; p=0.361) and matched populations. The higher proportion of patients undergoing general anaesthesia for TAVR with the PRO devices may reflect both the different time period analysed for the 2 groups and a centre-specific protocol, instead of a clinical need, since most of these procedures were performed in the same centres. The higher percentage of valve predilatation in the NEO2 group may reflect both the manufacturer’s recommendation for a systematic use, the low radial force of the valve, and the considerable proportion of patients with significant AV calcification in this group20. Nevertheless, the effect of predilatation is uncertain and its application did not have a significant impact on short-term adverse events in a subanalysis of the NEOPRO population10. Periprocedural complications were acceptable and similar in both groups. The higher proportion of minor vascular access complications in the PRO group may be partly explained by the different dimensions of the introducer sheaths between the PRO and NEO2 devices.
Both groups achieved high rates of predischarge VARC-3-defined intended performance of the valve (96.7% vs 96.3%; p=0.780). Moderate or severe PVL was a relevant concern in TAVR with the ACURATE neo bioprosthesis, with reported rates of up to 10%7825. In our study, the predischarge moderate or severe PVL rate after a NEO2 implantation was low (1.7%), with no reported cases of severe PVL, confirming the preliminary experiences with this device14. Concerning the Evolut devices, the rate of moderate or severe PVL has been progressively reduced from 8%-10% with the first-generation CoreValve to 0%-6% with the latest-generation Evolut PRO and PRO+ devices, which is in line with our results (4.1%)481726. A lower residual moderate or severe PVL in the NEO2 group in the overall population did not reach statistical significance after matching (2.0% vs 3.1%; p=0.281), indicating a comparable sealing performance of these devices after adjustment for baseline heterogeneity. Residual mild PVL was frequent (up to 39% in both groups). Since this has been associated with worse prognosis, we hope that future studies will further evaluate this issue27. The slightly lower mean gradients of the Evolut PRO bioprosthesis have been previously reported and may partly be explained by the more frequent use of the 29 mm device7. Nevertheless, the difference was not clinically relevant.
VARC-3 device success
VARC-3 device success at 30-day follow-up was acceptable in both groups (NEO2 84.3%, PRO 83.6%; p=0.688) (Central illustration). This finding was confirmed after adjustment for PS quintiles, in the PS-matched pairs, and was not influenced by AV calcification severity. Focusing on the 4 single components of the endpoint, the 30-day mortality rate was 2.5%, similar in the 2 groups and comparable with current reports; technical success was high, as previously discussed1416. In the overall population, few patients needed repeat intervention for valve dysfunction or cardiac structural complications. Nevertheless, patients undergoing TAVR with the PRO device more frequently experienced post-procedural vascular complications (12.7% vs 5.7%; p<0.001). This potential disadvantage was confirmed after adjustment for PS quintiles and in the matched pairs (Supplementary Table 2, Supplementary Table 5). This result may be partly explained by the low proportion of PRO+ devices in our registry (11.2% of the PRO group), which are delivered through a 2 Fr smaller sheath, and the higher AV calcification burden and NYHA Class in the PRO group, which may be a marker of overall frailty. Most of the difference was driven by minor vascular complications not requiring interventions, therefore not affecting device success. Finally, VARC-3-defined intended performance of the valve was high in both groups (Table 3).
To the best of our knowledge, the NEOPRO-2 registry is the first study comparing 30-day VARC-3-defined device success between the ACURATE neo2 and Evolut PRO/PRO+ devices. Our data are similar to the 30-day results from the Early Neo2 registry and the ACURATE neo2 CE Mark Study14. Considering the Evolut bioprostheses, the Medtronic TAVR 2.0 US Clinical Study reported 100% implant success and low 30-day mortality rates (1.7%) with the Evolut PRO device17. Satisfactory short-term outcomes emerged also in the FORWARD PRO study and in the STS/ACC TVT registry1628. Pending the results of ongoing trials (ClinicalTrials.gov: NCT03735667; NCT05036018), there is a lack of randomised data comparing the performance of these self-expanding THV. In this context, our study reports a similar rate of VARC-3 device success between the latest-generation NEO2 and PRO valves, also after adjustment for a range of baseline variables that may affect procedural outcomes.
VARC-3 early safety composite endpoint
The VARC-3 early safety composite endpoint at 30-day follow-up was achieved in 73.8% of patients, with higher rates after NEO2 implantation in the overall population (78.7% vs 71.3%; p<0.001) (Central illustration). Despite not achieving statistical significance, a trend towards higher 30-day early safety in the NEO2 group was observed after adjustment for PS quintiles (aOR 1.29, 95% CI: 0.97-1.71; p=0.082) and in the PS-matched cohort (77.1% vs 72.2%; p=0.095). This result was mainly driven by a lower incidence of new PPI at 30 days in the NEO2 group (overall population: 7.7% vs 15.6%; p<0.001), regardless of the degree of AV calcification, a finding that was confirmed also after adjustment for PS quintiles and in the PS-matched analysis. Therefore, our data confirm the favourable profile of the NEO2 device in terms of conduction disturbances, due to the reduced radial force and limited protrusion into the left ventricular outflow tract, compared to its precursor7829. With regard to the Evolut platform, the proportion of new PPI at 30 days in our registry (15.6%) is consistent with previous experiences, and we should acknowledge the absence of data on preprocedural conduction system diseases and implantation techniques7817. An "optimised" self-expanding valve (SEV) TAVR care pathway, including the cusp-overlap technique, is currently under evaluation in the ongoing Optimize PRO Study (ClinicalTrials.gov: NCT04091048). Since the TAVR period analysed for the PRO group started in 2017, our results may be influenced by a limited use of these novel approaches. In addition, 30-day data in the overall population suggested a reduced occurrence of moderate or severe PVL, vascular complications and bleedings in the NEO2 group. Potential explanations have been previously discussed for in-hospital outcomes.
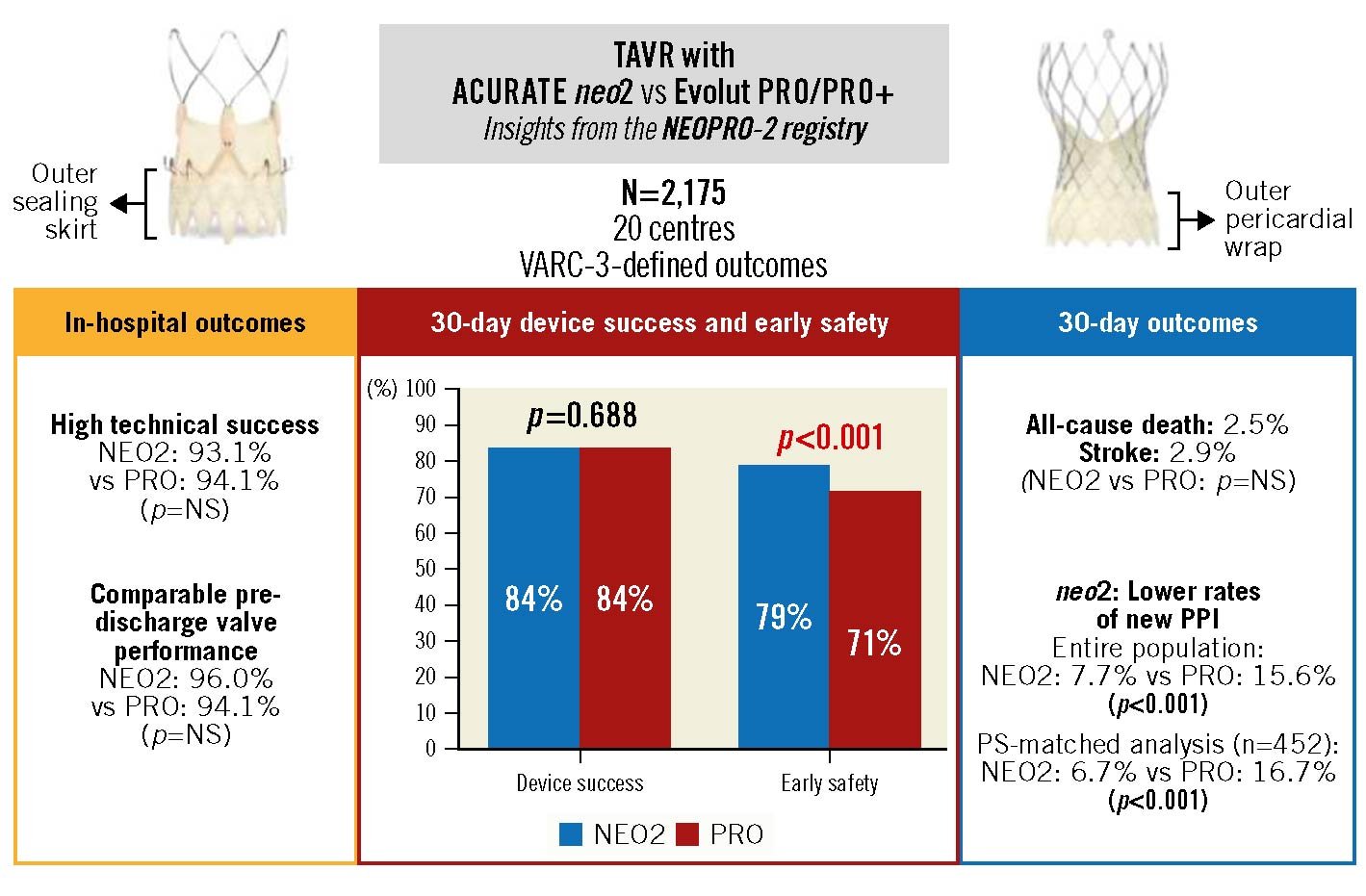
Central illustration. TAVR with NEO2 versus PRO/PRO+ devices in the NEOPRO-2 registry. Comparison of NEO2 and PRO/PRO+ devices for design characteristics, VARC-3-defined composite outcomes, and relevant single endpoints including all-cause death, stroke and new PPI. NEO2: ACURATE neo2; NS: not significant; PPI: permanent pacemaker implantation; PRO: Evolut PRO/PRO+; PS: propensity score; TAVR: transcatheter aortic valve replacement; VARC: Valve Academic Research Consortium
Limitations
Our study had a retrospective, observational design, with no core laboratory analysis of echocardiographic data or independent adjudication committee for clinical events. Follow-up data at 30 days were not available for all patients and hard events were numerically low. We performed PS adjustment, PS-matched comparison and a subgroup analysis based on the severity of AV calcification to overcome differences in baseline characteristics and potential confounders. However, a latent impact of unknown or unmeasured confounding factors cannot be excluded, including missing data on post-procedural medical therapy. We acknowledge that many centres contributed with nearly exclusively 1 valve type to the registry, adding potential selection and centre-specific bias which may not have been completely mitigated despite PS-matched and multivariable regression analyses. Furthermore, the different sample sizes between the NEO2 and PRO groups in the overall cohort may have influenced the study results. In addition, since the TAVR period analysed for the PRO group started in 2017, our results may not completely reflect the current performance of the PRO/PRO+ devices. Whether restriction of the comparison to a more recent time period may translate into different results is debatable. Finally, comparison with previous studies is complex, due to the heterogeneous populations included and the different endpoint definitions used and follow-up period assessed.
Conclusions
In our multicentre, contemporary, real-world registry, transfemoral TAVR with the ACURATE neo2 bioprosthesis achieved a short-term performance comparable with the Evolut PRO/PRO+ THV in terms of VARC-3-defined outcomes, reflecting current TAVR standards with new-generation self-expanding devices. A tendency for higher VARC-3 early safety in the NEO2 group was mainly driven by reduced rates of new PPI. Randomised studies are needed to confirm our exploratory findings.
Impact on daily practice
In the real-world, multicentre NEOPRO-2 registry, transfemoral TAVR with the new-generation ACURATE neo2 (NEO2) bioprosthesis achieved a short-term performance similar to the Evolut PRO/PRO+ platform in terms of VARC-3-defined outcomes and low rates of predischarge more-than-mild paravalvular leak (1.7%), meeting current TAVR standards with self-expanding devices. Our data may suggest a higher VARC-3 early safety in the NEO2 group, driven mainly by reduced rates of new permanent pacemaker implantation. While randomised studies are needed to confirm our exploratory analysis, these real-world results may be considered as a further step towards tailoring valve selection in TAVR candidates.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
W-K. Kim is a proctor for Boston Scientific and Abbott Vascular; and has received personal fees from Abbott Laboratories, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril outside the submitted work. M. Barbanti has served as a consultant for Edwards Lifesciences. M. Adamo has received consultation and speaker fees from Abbott Vascular and Medtronic. R. Estévez-Loureiro has served as a consultant for Abbott Vascular, Boston Scientific, and Edwards Lifesciences. F. De Marco has served as a proctor for Boston Scientific and Kardia. S. Toggweiler is a proctor and consultant for Boston Scientific, Medtronic, and Biosensors/NVT; is a proctor for Abbott Vascular; and is a consultant for Medira, Shockwave, Teleflex, AtHeart, VeoSource, and Equity in Hi-D Imaging. V. Veulemans has received lecture fees and travel support from Medtronic and Edwards Lifesciences. D. Mylotte is a proctor for Medtronic and Microport. B. Reimers has received speaker honoraria from Boston Scientific. L. Sondergaard has received consultant fees and/or institutional research grants from Abbott Vascular, Boston Scientific, Edwards Lifesciences, Medtronic, and SMT. A. Wolf is a proctor for Medtronic and Boston Scientific. A. Latib has served on advisory boards or as a consultant for Medtronic, Boston Scientific, Philips, Edwards Lifesciences, and Abbott Vascular. A. Mangieri has received a research grant (to the institution) from Boston Scientific; and has served on a medical advisory board for Boston Scientific. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer. The other authors have no conflicts of interest to declare relevant to the contents of this paper.
Supplementary data
To read the full content of this article, please download the PDF.

