Abstract
BACKGROUND: There is a lack of comparative data on transcatheter aortic valve implantation (TAVI) in degenerated surgical prostheses (valve-in-valve [ViV]).
AIMS: We sought to compare outcomes of using two self-expanding transcatheter heart valve (THV) systems for ViV.
METHODS: In this retrospective multicentre registry, we included consecutive patients undergoing transfemoral ViV using either the ACURATE neo/neo2 (ACURATE group) or the Evolut R/PRO/PRO+ (EVOLUT group). The primary outcome measure was technical success according to Valve Academic Research Consortium (VARC)-3. Secondary outcomes were 30-day all-cause mortality, device success (VARC-3), coronary obstruction (CO) requiring intervention, rates of severe prosthesis-patient mismatch (PPM), and aortic regurgitation (AR) ≥moderate. Comparisons were made after 1:1 propensity score matching.
RESULTS: The study cohort comprised 835 patients from 20 centres (ACURATE n=251; EVOLUT n=584). In the matched cohort (n=468), technical success (ACURATE 92.7% vs EVOLUT 88.9%; p=0.20) and device success (69.7% vs 73.9%; p=0.36) as well as 30-day mortality (2.8% vs 1.6%; p=0.392) were similar between the two groups. The mean gradients and rates of severe PPM, AR ≥moderate, or CO did not differ between the groups. Technical and device success were higher for the ACURATE platform among patients with a true inner diameter (ID) >19 mm, whereas a true ID ≤19 mm was associated with higher device success − but not technical success − among Evolut recipients.
CONCLUSIONS: ViV TAVI using either ACURATE or Evolut THVs showed similar procedural outcomes. However, a true ID >19 mm was associated with higher device success among ACURATE recipients, whereas in patients with a true ID ≤19 mm, device success was higher when using Evolut.
Surgical bioprostheses are prone to degeneration and failure. Over the last few years, transcatheter aortic valve implantation (TAVI) within failed surgical bioprostheses (referred to as valve-in-valve [ViV]) has become an established treatment option. Although less invasive than surgical reoperation, these percutaneous procedures require careful patient selection and detailed preprocedural planning including multidetector computed tomography (MDCT). Moreover, certain procedural risks, including a higher risk of coronary obstruction (CO), valve malpositioning, and elevated transprosthetic gradients along with prosthesis-patient mismatch (PPM) after the procedure, need to be considered12. Selection of transcatheter heart valves (THV) for ViV procedures remains a matter of debate. Self-expanding (SE) THV systems with a supra-annular design have proven to exhibit more favourable haemodynamics than intra-annular balloon-expandable (BE) valves, whereas rates of permanent pacemaker implantation (PPI) were shown to be higher among SE devices3. Among SE THVs, the Evolut (Medtronic) valve represents the most used platform for this specific indication, as it is resheathable and can be repositioned easily. The ACURATE neo (Boston Scientific) valve is another supra-annular SE device that may be well suited for ViV because of low PPI rates and its open frame design which, along with commissural alignment, may facilitate coronary reaccess4. While the feasibility of ViV using the ACURATE neo THV was demonstrated previously in a small registry5, no data exist that compare the ACURATE and Evolut THV platforms for ViV TAVI. The purpose of this study was to compare these two SE THV platforms regarding procedural and clinical outcomes.
Methods
STUDY COHORT
In the AVENGER study (Acurate Versus Evolut multiceNter reGistry for valvE-in-valve theRapy), consecutive patients who underwent transfemoral ViV TAVI for severe structural valve degeneration in participating centres between 2012 and 2023 using either the Evolut R/PRO/PRO+ valves (EVOLUT group; Medtronic) or the ACURATE neo/neo2 (ACURATE group; Boston Scientific) were analysed retrospectively. Descriptions of the devices and the implantation techniques have been published previously67. All patients were discussed within the local Heart Team in adherence to guidelines on valvular heart disease8910. The valve selection was at the discretion of the operator. Patients were treated in a hybrid operating suite under either conscious sedation or general anaesthesia according to local standard practice. Data collection included baseline characteristics, echocardiography and MDCT data, procedural characteristics, and in-hospital as well as 30-day clinical outcomes. In addition, data from the last available clinical follow-up were gathered. Data were consolidated in a joint database for statistical analyses, and inconsistencies in the data were resolved by direct communication with the investigator of each participating centre. The study was approved by local ethics committees of the participating centres and complied with the Declaration of Helsinki.
DEFINITIONS
The label, type (stented bioprosthesis with leaflets mounted internally, stented bioprosthesis with leaflets mounted externally, stentless bioprosthesis), and size (true inner diameter [ID]) of the failed surgical aortic valve (SAV)11 were recorded as well as the time from surgery to ViV and the mode of failure (stenosis, regurgitation, combined).
A preprocedural MDCT of the entire aorta and ilio-femoral arteries was performed and analysed according to local standard methods12. Also determined were the distances from the bioprosthetic sewing ring to the left and right coronary arteries (LCA, RCA), the virtual THV-to-coronary distance (VTC) to the LCA and RCA, the width of the sinotubular junction (STJ), and the diameter of the sinus of Valsalva (SOV)13. The cover index (CI) was calculated as CI=100*(prosthesis size-annulus diameter)/prosthesis size.
The risk of CO was categorised according to the type of prosthesis and VTC (Supplementary Table 1) as follows1:
Low risk of CO:
- Stented prosthesis with internally mounted leaflets
- Stentless prosthesis and VTC ≥4 mm
Intermediate risk of CO:
- Stented prosthesis with externally mounted leaflets and VTC ≥4 mm
- Stented prosthesis with externally mounted leaflets/stentless prosthesis (VTC unspecified)
High risk of CO:
- Stented prosthesis with externally mounted leaflets/stentless prosthesis and VTC <4 mm
CO was defined as an obstruction of a coronary ostium requiring intervention, including the deployment of pre-emptively placed coronary stents.
The implantation depth of the prosthesis was determined upon final angiography at the site of the former non-coronary cusp and defined as the distance between the ventricular aspect of the bioprosthetic ring and the inflow segment of the THV (Figure 1). Malpositioning was defined as incorrect positioning of the device (too high: implantation depth <0 mm; too low: implantation depth ≥10 mm) requiring intervention − including the implantation of a second valve or surgical valve replacement − or leading to valve dysfunction (aortic regurgitation [AR] ≥moderate or mean gradient ≥20 mmHg).
Postprocedural AR was assessed prior to discharge by transthoracic echocardiography using a 3-class grading scheme (none/trace, mild, moderate, severe) in adherence to existing recommendations14. PPM was defined according to Valve Academic Research Consortium (VARC)-3 criteria14. Using the available reference values of the normal effective orifice area (EOA) for each given SAV model and size indexed for body surface area, the projected indexed EOA and PPM of the failed SAV were determined15.
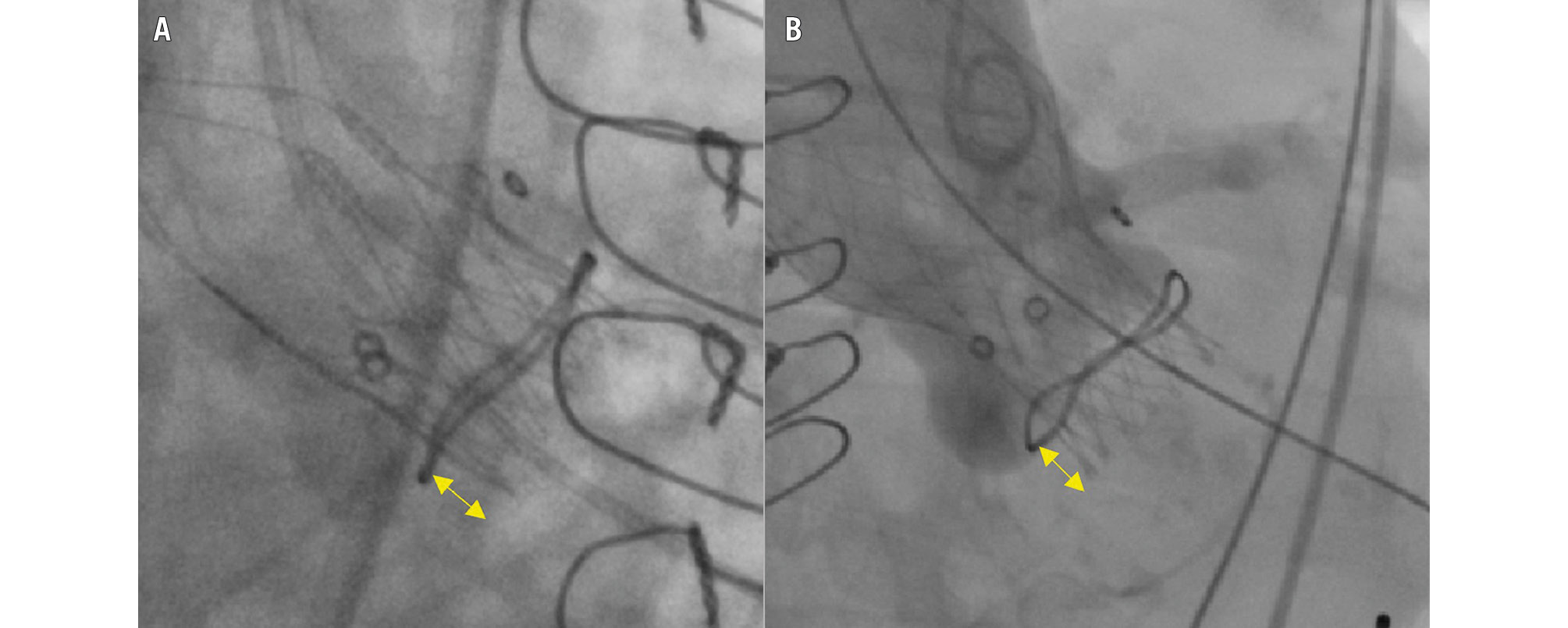
Figure 1. Implantation depth measurements. A) ACURATE and B) EVOLUT. The double-headed arrows denote the implantation depth at the non-coronary cusp. ACURATE: ACURATE neo/neo2 group; EVOLUT: Evolut R/PRO/PRO+ group
OUTCOMES OF INTEREST
The primary outcome measure was technical success according to VARC-3 criteria. Secondary outcome measures were 30-day all-cause mortality and device success at 30 days according to VARC-3 consensus, including technical success, freedom from mortality, freedom from surgery or intervention related to the device or to a major vascular- or access-related or cardiac structural complication, and intended performance of the valve14. Further secondary endpoints were CO, new PPI, severe PPM, and AR ≥moderate upon discharge.
STATISTICAL ANALYSIS
For the analysis, the patient population was divided into two groups based on the implanted THV platform. Furthermore, THVs were categorised into early (ACURATE neo, Evolut R) and new (ACURATE neo2, Evolut PRO/PRO+) generation. To capture the different available THV sizes between the two groups, we categorised them into small (23 mm), medium (25-26 mm), and large (27-34 mm). A true ID ≤19 mm was defined as a small SAV.
Continuous data are presented as median and interquartile range [IQR], and categorical data are provided as counts with percentages. Comparisons of the groups were performed using the Mann-Whitney U test and the two-sided Fisher’s exact or the Chi-squared test, as appropriate. Subgroup and logistic regression analyses were performed for technical success and device success. For the logistic regression analysis, we stratified success rates according to the valve type and included variables with p-value≤0.1 in the univariate analysis or with clinical relevance. A two-sided p-value<0.05 was considered to indicate statistical significance.
To account for differences in baseline characteristics and the effect of a potential selection bias, a one-to-one propensity score matching (PSM) was performed using the R package “MatchIt”, version 4.5.4 (R Foundation for Statistical Computing). Missing baseline data were imputed using the predictive mean matching function (R package “Mice”, version 3.16.0). The following variables showing significant univariate differences between both groups or those with known influence on outcome were included in the matching algorithm: EOA, type of SAV, true ID, STJ, SOV, and CO risk category (Supplementary Table 2). All other analyses were performed with STATA IC, version 18.1 (StataCorp).
Results
BASELINE CHARACTERISTICS
The study population consisted of 835 patients from 20 international centres undergoing ViV TAVI using the ACURATE (n=251) or Evolut (n=584) platform. The average age was 79 [IQR 74; 83] years, and 46.5% were female. All baseline characteristics were similar between the two groups. The EOA was larger in the EVOLUT group. In the ACURATE group, the proportion of stented SAVs with externally mounted leaflets was higher, whereas the proportion of stentless SAVs was lower. Supplementary Table 2 provides an overview of the failed SAVs.
The coronary distance to the LCA, the VTC to the LCA, the VTC to the RCA, and the SOV diameter were smaller among ACURATE recipients, who therefore exhibited a higher risk of CO. Further details are shown in Table 1.
Table 1. Baseline characteristics and computed tomography measurements.
| Variable | ACURATE (n=251) | EVOLUT (n=584) | p-value | ACURATE (n=234) | EVOLUT (n=234) | p-value |
|---|---|---|---|---|---|---|
| Age, years | 79.0 [74.3; 84.0] | 79.0 [74.0; 83.0] | 0.664 | 79.0 [74.1; 83.0] | 79.0 [73.7; 82.3] | 0.343 |
| Female sex | 112 (44.6) | 276 (47.3) | 0.483 | 107 (45.7) | 110 (47.0) | 0.853 |
| Body mass index, kg/m2 | 26.2 [23.2; 29.4] | 26.2 [23.8; 30.1]; 581 | 0.355 | 26.2 [23.4; 29.4] | 26.0 [23.7; 29.4] | 0.966 |
| EuroSCORE II, % | 8.6 [6.1; 13.6] | 8.6 [5.5; 12.6] | 0.198 | 8.6 [6.0; 13.2] | 8.3 [5.1; 12.8] | 0.194 |
| STS score, % | 4.3 [2.7; 6.6]; 107 | 4.4 [2.8; 6.7]; 262 | 0.697 | 4.3 [2.7; 6.6] | 4.2 [2.9; 7.1] | 0.656 |
| Risk category | 0.349 | 0.129 | ||||
| Low risk | 19 (7.6) | 63 (10.8) | 17 (7.3) | 30 (12.8) | ||
| Intermediate risk | 92 (36.7) | 202 (34.6) | 86 (36.8) | 84 (35.9) | ||
| High risk | 140 (55.8) | 319 (54.6) | 131 (56.0) | 120 (51.3) | ||
| eGFR, ml/min/1.73 m2 | 55.0 [40.0; 75.9] | 53.7 [40.5; 70.0] | 0.443 | 55.0 [40.0; 75.8] | 53.0 [41.0; 69.8] | 0.456 |
| Coronary artery disease | 137 (54.6) | 356 (61.0) | 0.086 | 127 (54.3) | 140 (59.8) | 0.262 |
| Prior pacemaker | 44 (17.5) | 95 (16.3) | 0.653 | 41 (17.5) | 41 (17.5) | >0.99 |
| Atrioventricular block I° | 43/243 (17.7) | 74/356 (20.8) | 0.349 | 42 (17.9) | 45 (19.2) | 0.812 |
| Right bundle branch block | 19/244 (7.8) | 47/411 (11.4) | 0.134 | 19 (8.1) | 22 (9.4) | 0.744 |
| Ejection fraction, % | 57.0 [45.0; 60.0] | 55.0 [45.0; 60.0]; 577 | 0.385 | 57.0 [48.3; 60.0] | 56.0 [45.3; 60.0] | 0.635 |
| Mean gradient, mmHg | 39.0 [23.0; 49.0]; 241 | 36.0 [24.0; 46.0]; 558 | 0.146 | 39.0 [23.0; 49.0] | 36.0 [23.0; 46.0] | 0.272 |
| Effective orifice area, cm2 | 0.7 [0.6; 1.0]; 209 | 0.8 [0.7; 1.0]; 493 | 0.031 | 0.8 [0.6; 1.0] | 0.8 [0.7; 1.0] | 0.259 |
| Aortic regurgitation baseline | 0.926 | 0.728 | ||||
| None/trace | 61 (24.7) | 148 (25.5) | 55 (23.8) | 49 (20.9) | ||
| Mild | 70 (28.3) | 162 (27.9) | 67 (29.0) | 73 (31.2) | ||
| Moderate | 38 (15.4) | 97 (16.7) | 37 (16.0) | 44 (18.8) | ||
| Severe | 78 (31.6) | 174 (29.9) | 72 (31.2) | 68 (29.1) | ||
| Type of surgical prosthesis | 0.022 | 0.880 | ||||
| Stented, leaflets internal | 150 (69.8) | 381 (65.2) | 149 (63.7) | 154 (65.8) | ||
| Stented, leaflets external | 89 (35.5) | 157 (26.9) | 75 (32.1) | 70 (29.9) | ||
| Stentless | 12 (4.8) | 46 (7.9) | 10 (4.3) | 10 (4.3) | ||
| Mode of failure | 0.384 | 0.881 | ||||
| Stenosis | 131 (52.2) | 303 (51.9) | 122 (52.1) | 119 (50.9) | ||
| Regurgitation | 37 (14.7) | 68 (11.6) | 32 (13.7) | 30 (12.8) | ||
| Mixed | 80 (33.3) | 213 (36.5) | 80 (34.2) | 85 (36.3) | ||
| Time from surgery to failure, years | 10.4 [7.0; 13.0]; 249 | 10.0 [7.0; 13.0]; 580 | 0.359 | 10.6 [7.2; 13.0] | 10.0 [8.0; 13.0] | 0.533 |
| True ID, mm | 21.0 [19.0; 23.0]; 250 | 21.0 [19.0; 22.0]; 580 | 0.882 | 21.0 [19.0; 21.8] | 21.0 [19.0; 21.0] | 0.936 |
| True ID ≤19 mm | 104 (41.6) | 234 (40.3) | 0.736 | 94 (40.3) | 92 (39.5) | 0.925 |
| Severe pre-existing projected PPM | 5/226 (2.2) | 20/511 (3.9) | 0.239 | 4 (1.9) | 10 (4.8) | 0.172 |
| Sinotubular junction, mm | 29.0 [26.2; 32.7]; 200 | 29.0 [26.6; 32.0]; 199 | 0.830 | 29.2 [26.3; 32.6] | 28.9 [26.6; 32.9] | 0.836 |
| Sinus of Valsalva, mm | 31.4 [28.6; 35.0]; 213 | 32.5 [29.2; 36.0]; 283 | 0.013 | 31.7 [29.0; 34.9] | 31.4 [29.0; 35.6] | 0.737 |
| Distance to LCA, mm | 8.4 [5.9; 11.6]; 230 | 9.3 [7.0; 12.8]; 344 | 0.004 | 8.4 [6.0; 11.6] | 9.1 [6.8; 12.6] | 0.141 |
| Distance to RCA, mm | 12.3 [8.9; 15.7]; 230 | 12.7 [9.9; 15.7]; 342 | 0.297 | 12.6 [9.2; 15.7] | 12.4 [9.7; 16.0] | 0.796 |
| VTC (LCA), mm | 5.3 [3.9; 6.6]; 210 | 5.7 [4.6; 7.0]; 269 | 0.001 | 5.5 [4.0; 6.6] | 5.6 [4.4; 6.9] | 0.227 |
| VTC (RCA), mm | 4.7 [3.5; 6.2]; 204 | 5.2 [4.0; 6.7]; 239 | 0.002 | 4.8 [3.6; 6.2] | 5.2 [3.8; 6.9] | 0.174 |
| Coronary obstruction risk category | <0.001 | 0.926 | ||||
| Low risk | 154 (61.4) | 394 (67.5) | 153 (65.4) | 157 (67.1) | ||
| Intermediate risk | 58 (23.1) | 163 (27.9) | 58 (24.8) | 55 (23.5) | ||
| High risk | 39 (15.5) | 27 (4.6) | 23 (9.8) | 22 (9.4) | ||
| Values denote n (%), n/N (%), or median [IQR]. ACURATE: ACURATE neo/neo2 group; eGFR: estimated glomerular filtration rate; EuroSCORE: European System for Cardiac Operative Risk Evaluation; EVOLUT: Evolut R/PRO/PRO+ group; ID: internal diameter; IQR: interquartile range; LCA: left coronary artery; PPM: prosthesis-patient mismatch; RCA: right coronary artery; STS: Society of Thoracic Surgeons; THV: transcatheter heart valve; VTC: virtual THV-to-coronary distance | ||||||
PROCEDURAL CHARACTERISTICS AND CLINICAL OUTCOMES
Details of procedural characteristics are provided in Table 2. In the ACURATE group, the implanted THV sizes were smaller, the rates of predilatation and post-dilatation were higher, the procedural duration and fluoroscopy time were shorter, the amount of contrast agent used was smaller, the use of cerebral protection was less frequent, and the implantation depth was smaller than in the EVOLUT group. Implantation with patient-specific commissural alignment and employment of coronary protection was more common in the ACURATE group. The latter included wire protection in 8.0% vs 4.5%, stent protection in 11.6% vs 10.6%, and BASILICA in 0.4% vs 2.1%.
Technical success (ACURATE 91.2% vs EVOLUT 89.2%; p=0.454) and device success (68.1% vs 68.8%; p=0.871) were similar between the groups (Table 3). Reasons for device failure are listed in Supplementary Table 3. Rates of AR ≥moderate, severe PPM, and mean gradients did not differ between the groups. The EOA was significantly smaller in the ACURATE group (ACURATE 1.5 [IQR 1.3; 1.7] vs EVOLUT 1.5 [IQR 1.3; 1.9] cm2; p=0.021). The need for PPI was very low overall and was numerically lower in the ACURATE group (0.8% vs 2.9%; p=0.060).
In the matched cohort (234 pairs), all baseline variables and EOA, type of SAV, and CO risk criteria were well balanced between the two groups (Table 1). The use of coronary protection was similar, whereas most other procedural characteristics were different (Table 2). Despite having the same median value, the EOA post-intervention was larger in the EVOLUT group (1.5 [1.4;2.0] vs 1.5 [1.3;1.7] cm²; p=0.022). All other procedural outcomes were similar between the two groups (Table 3).
Figure 2 displays the projected indexed EOA versus the measured indexed EOA which was smaller than expected in the ACURATE group and larger than expected in the EVOLUT group, but the differences were small and not statistically significant.
Table 2. Procedural data.
| Variable | ACURATE (n=251) | EVOLUT (n=584) | p-value | ACURATE (n=234) | EVOLUT (n=234) | p-value |
|---|---|---|---|---|---|---|
| Prosthesis size | <0.001 | <0.001 | ||||
| Small (23 mm) | 195 (77.7) | 291 (49.8) | 180 (76.9) | 108 (46.2) | ||
| Medium (25, 26 mm) | 47 (18.7) | 234 (40.1) | 45 (19.2) | 105 (44.9) | ||
| Large (27, 29, 34 mm) | 9 (3.6) | 59 (10.1) | 9 (3.8) | 21 (9.0) | ||
| Cover index, % | 8.7 [8.7; 17.4] | 17.4 [11.5; 19.2] | <0.001 | 8.7 [8.7; 17.4] | 17.4 [13.8; 19.2] | <0.001 |
| Early-generation device | 132 (52.6) | 569 (97.4) | <0.001 | |||
| Procedural duration, min | 53 [37;80]; 236 | 74 [55; 105]; 544 | <0.001 | 52 [36; 80] | 74 [56; 100] | <0.001 |
| Fluoroscopy time, min | 12.4 [9.0; 20.3]; 234 | 18 [12.6; 27.5]; 541 | <0.001 | 12.3 [9.0; 19.5] | 18.1 [13.3; 27.1] | <0.001 |
| Contrast agent, ml | 60 [25; 98]; 245 | 95 [50; 146]; 571 | <0.001 | 60 [22; 95] | 96 [50; 140] | <0.001 |
| Predilatation | 111 (44.4) | 42 (7.2) | 0.037 | 102 (43.6) | 19 (8.1) | <0.001 |
| Post-dilatation | 144 (57.4) | 289 (49.5) | 0.195 | 136 (58.1) | 115 (49.1) | 0.064 |
| Attempted BVF | 9 (3.6) | 12 (2.1) | 0.067 | 9 (3.8) | 5 (2.1) | 0.417 |
| BVF timing at predilatation | 8/9 (88.9) | 5/12 (41.7) | 0.171 | 8/9 (88.9) | 1/5 (20.0) | 0.023 |
| BVF successful | 7/9 (77.8) | 12/12 (100) | 0.119 | 7/9 (77.8) | 5/5 (100) | 0.505 |
| Haemodynamic compromise after BVF | 4/9 (44.4) | 1/12 (8.3) | <0.001 | 4/9 (44.4) | 1/5 (20.0) | 0.580 |
| Cerebral protection | 19 (7.6) | 186 (31.9) | <0.001 | 18 (7.7) | 70 (29.9) | <0.001 |
| Commissural alignment | 98 (39.0) | 15 (2.6) | 0.111 | 91 (38.9) | 8 (3.4) | <0.001 |
| Coronary protection | 49 (19.5) | 88 (15.1) | <0.001 | 40 (17.1) | 44 (18.8) | 0.186 |
| Implantation depth, mm | 2.5 [1.0; 4.5]; 240 | 4.7 [2.7; 6.4]; 468 | <0.001 | 2.0 [1.0; 4.2] | 4.0 [3.0; 6.0] | <0.001 |
| Values denote n (%), n/N (%), median [IQR]. ACURATE: ACURATE neo/neo2 group; BVF: bioprosthetic valve fracturing; EVOLUT: Evolut R/PRO/PRO+ group; IQR: interquartile range | ||||||
Table 3. In-hospital outcomes and complications.
| Variable | ACURATE (n=251) | EVOLUT (n=584) | p-value | ACURATE (n=234) | EVOLUT (n=234) | p-value |
|---|---|---|---|---|---|---|
| Mean gradient post-intervention, mmHg | 13.0 [9.0; 19.0]; 249 | 13.0 [9.0; 18.0]; 561 | 0.376 | 13.0 [9.0; 19.0] | 12.0 [9.0; 16.0] | 0.193 |
| EOA post-intervention, cm2 | 1.5 [1.3; 1.7]; 187 | 1.5 [1.3; 1.9]; 250 | 0.021 | 1.5 [1.3; 1.7] | 1.5 [1.4; 2.0] | 0.022 |
| Severe PPM | 30/181 (16.6) | 35/249 (14.1) | 0.472 | 28 (16.6) | 10 (9.8) | 0.149 |
| AR ≥moderate | 6/249 (2.4) | 17/574 (3.0) | 0.659 | 6 (2.6) | 4 (1.7) | 0.532 |
| Technical success (VARC-3) | 229 (91.2) | 521 (89.2) | 0.375 | 217 (92.7) | 208 (88.9) | 0.200 |
| Device success (VARC-3) | 171 (68.1) | 402 (68.8) | 0.840 | 163 (69.7) | 173 (73.9) | 0.355 |
| Coronary occlusion requiring PCI | 13 (5.2) | 24 (4.1) | 0.491 | 9 (3.8) | 11 (4.7) | 0.820 |
| Myocardial infarction | 1 (0.4) | 4 (0.7) | 0.623 | 0 | 2 (0.9) | 0.499 |
| Annular rupture | 0 | 1 (0.2) | 0.512 | 0 | 1 (0.4) | >0.99 |
| Conversion to sternotomy | 1 (0.4) | 2 (0.3) | 0.901 | 1 (0.4) | 0 | >0.99 |
| Multiple valve implantation | 4 (1.6) | 8 (1.4) | 0.803 | 4 (1.7) | 4 (1.7) | >0.99 |
| Malpositioning | 6 (3.1) | 18 (3.1) | 0.936 | 7 (3.0) | 5 (2.1) | 0.771 |
| Major vascular complication | 14 (5.6) | 36 (6.2) | 0.743 | 12 (5.1) | 11 (4.7) | >0.99 |
| Type 3-4 bleeding | 10 (4.2) | 30 (5.1) | 0.474 | 8 (3.4) | 13 (5.6) | 0.372 |
| Any stroke | 4 (1.6) | 11 (1.9) | 0.770 | 4 (1.7) | 6 (2.6) | 0.751 |
| Acute kidney injury stage 3-4 | 5 (2.0) | 23 (4.0) | 0.152 | 4 (1.7) | 6 (2.6) | 0.751 |
| Pacemaker implantation | 2 (0.8) | 17 (2.9) | 0.060 | 2 (0.9) | 6 (2.6) | 0.285 |
| Values denote n (%), n/N (%), or median [IQR]. ACURATE: ACURATE neo/neo2 group; AR: aortic regurgitation; EOA: effective orifice area; EVOLUT: Evolut R/PRO/PRO+ group; IQR: interquartile range; PCI: percutaneous coronary intervention; PPM: prosthesis-patient mismatch; VARC: Valvular Academic Research Consortium | ||||||
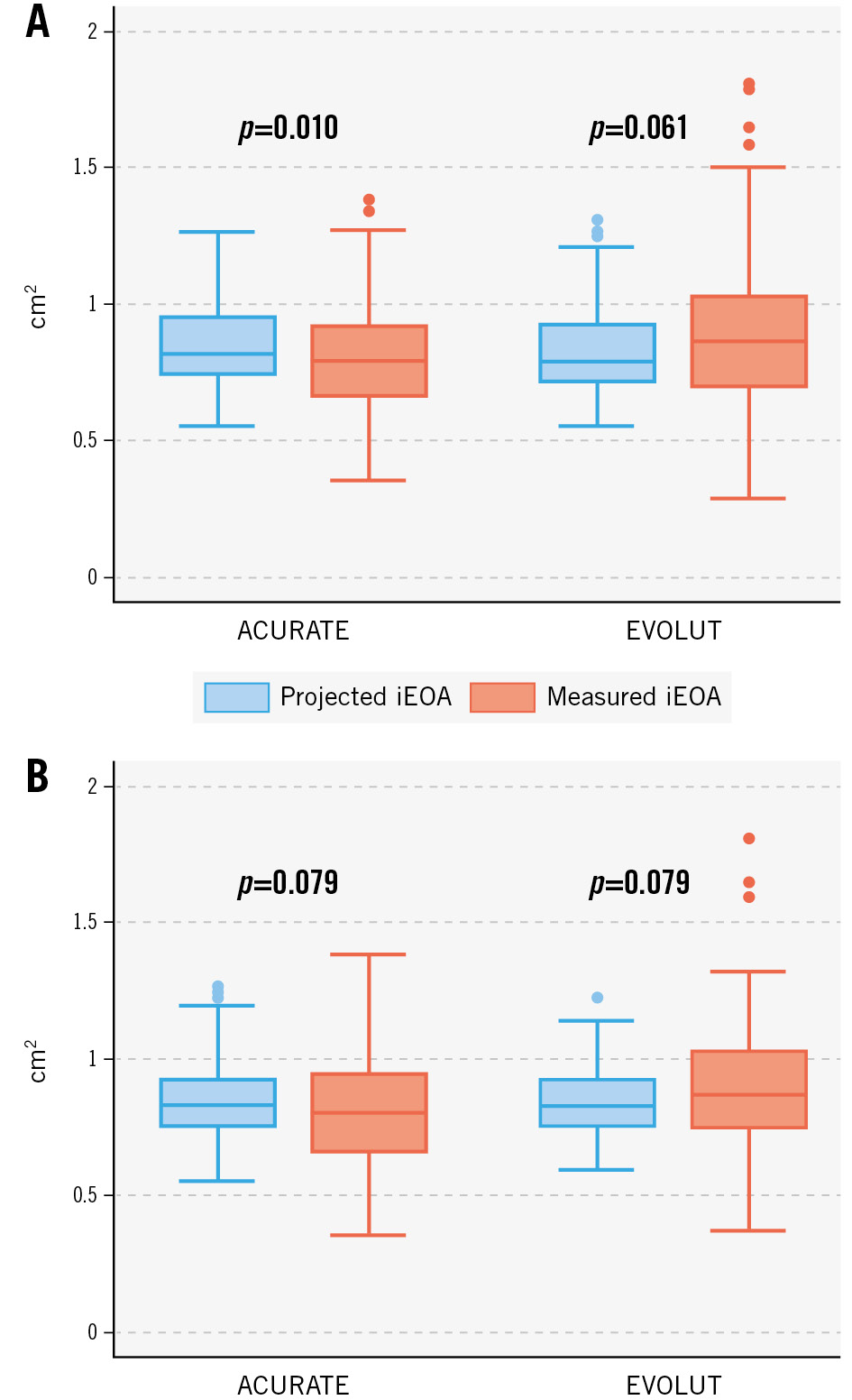
Figure 2. Projected and measured indexed effective orifice area. Projected and measured indexed effective orifice area (iEAO) in the unmatched (A) and matched (B) cohorts.ACURATE: ACURATE neo/neo2 group; EVOLUT: Evolut R/PRO/PRO+ group
SUBGROUP AND PREDICTOR ANALYSES
Results of subgroup analyses are depicted in Figure 3. A true ID >19 mm was associated with higher rates of technical success and device success for the ACURATE platform when compared with the Evolut platform, whereas in patients with a true ID ≤19 mm, device success – but not technical success – was higher among Evolut recipients.
In the subgroup of patients with predominant aortic regurgitation as the failure mode, technical success and device success were higher among ACURATE recipients. All other subgroup analyses did not show any differences between the two platforms.
Supplementary Table 4 and Supplementary Table 5 summarise the logistic regression analysis for technical success and device success stratified for each valve platform. Independent predictors of technical failure were a smaller implantation depth and the use of externally mounted or stentless SAVs for the ACURATE platform, whereas for the Evolut platform, there was no independent predictor. Independent predictors of device failure were a smaller true ID and the use of stentless SAVs for the ACURATE platform, whereas for the EVOLUT platform, a smaller implantation depth and a smaller true ID predicted device failure.
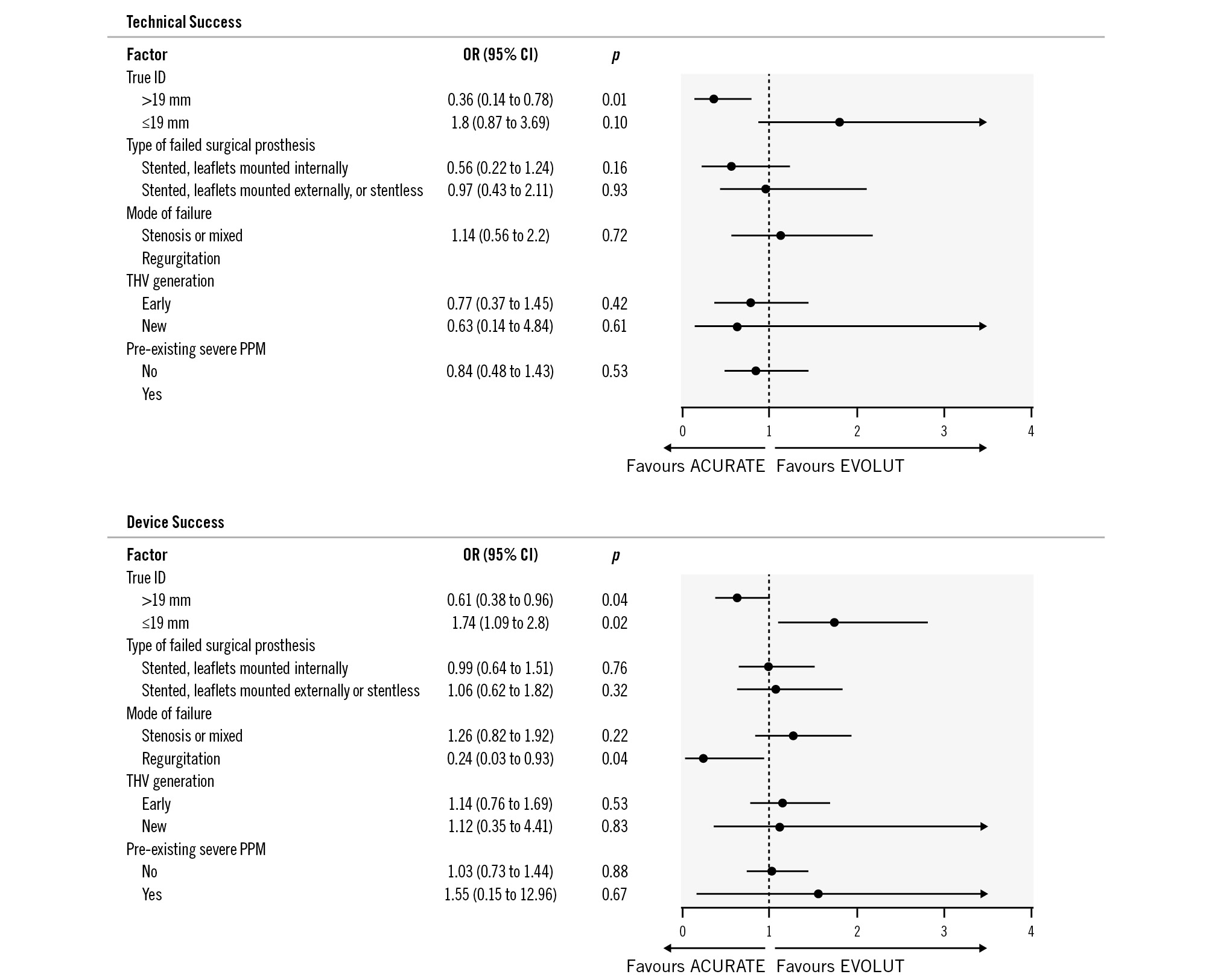
Figure 3. Subgroup analyses in the total cohort comparing ACURATE vs Evolut platforms for technical and device success. A) Technical success was more likely when using the ACURATE platform in cases with a true inner diameter (true ID) >19 mm and when the mode of failure was aortic regurgitation (the odds ratio was not calculated due to a 100% success rate for ACURATE vs 85.3% for Evolut). B) Among patients with a true ID ≤19 mm, device success was higher for Evolut, whereas among patients with a true ID >19 mm, device success was higher for ACURATE. ACURATE: ACURATE neo/neo2 group; CI: confidence interval; EVOLUT: Evolut R/PRO/PRO+ group; ID: inner diameter; OR: odds ratio; PPM: prosthesis-patient mismatch; THV: transcatheter heart valve
CORONARY OCCLUSION
Periprocedural CO occurred in a total of 37 cases (4.4%) and was similar between the two groups. All COs occurred in the second half of the study period (after the year 2016; 37/784 [4.7%] vs 0/51 [0%]; p=0.092). Among the patients with CO, coronary protection had been employed in 30 (90.1%) cases (11/13 [84.6%] in the ACURATE vs 19/24 [79.2%] in the EVOLUT group), whereas in 7 (18.9%) cases, no coronary protection had been employed (ACURATE 2/13 [15.4%] vs EVOLUT 5/24 [20.8%]; p=0.896). In the subset of patients without coronary protection, coronary access issues leading to failed percutaneous coronary intervention were noted in 3 of 7 cases (ACURATE n=1 vs EVOLUT n=2). Figure 4 shows that the incidence of CO was similar for the two THV platforms across different categories of risk of CO.
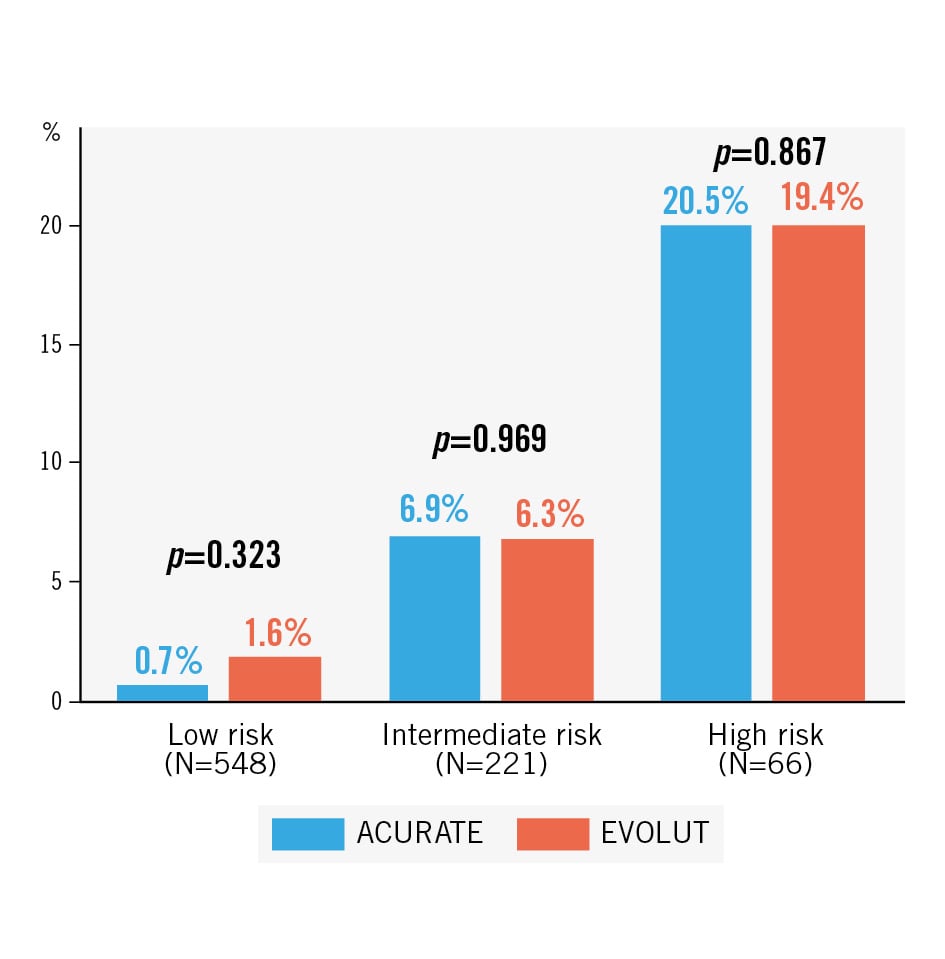
Figure 4. Coronary occlusion rates according to risk categories in the unmatched cohort. Across the different risk categories, coronary occlusion rates were similar between ACURATE and EVOLUT groups. ACURATE: ACURATE neo/neo2 group; EVOLUT: Evolut R/PRO/PRO+ group
SURVIVAL DATA
The median follow-up time was 11 [IQR 1; 20] months. At 30 days, all-cause mortality was similar between the two groups both in the unmatched (2.9% vs 1.9%; p=0.429) and in the matched cohorts (2.8% vs 1.6%; p=0.392). Figure 5 shows Kaplan-Meier curves of survival stratified according to groups; there was no difference in all-cause mortality up to 24 months (Central illustration).
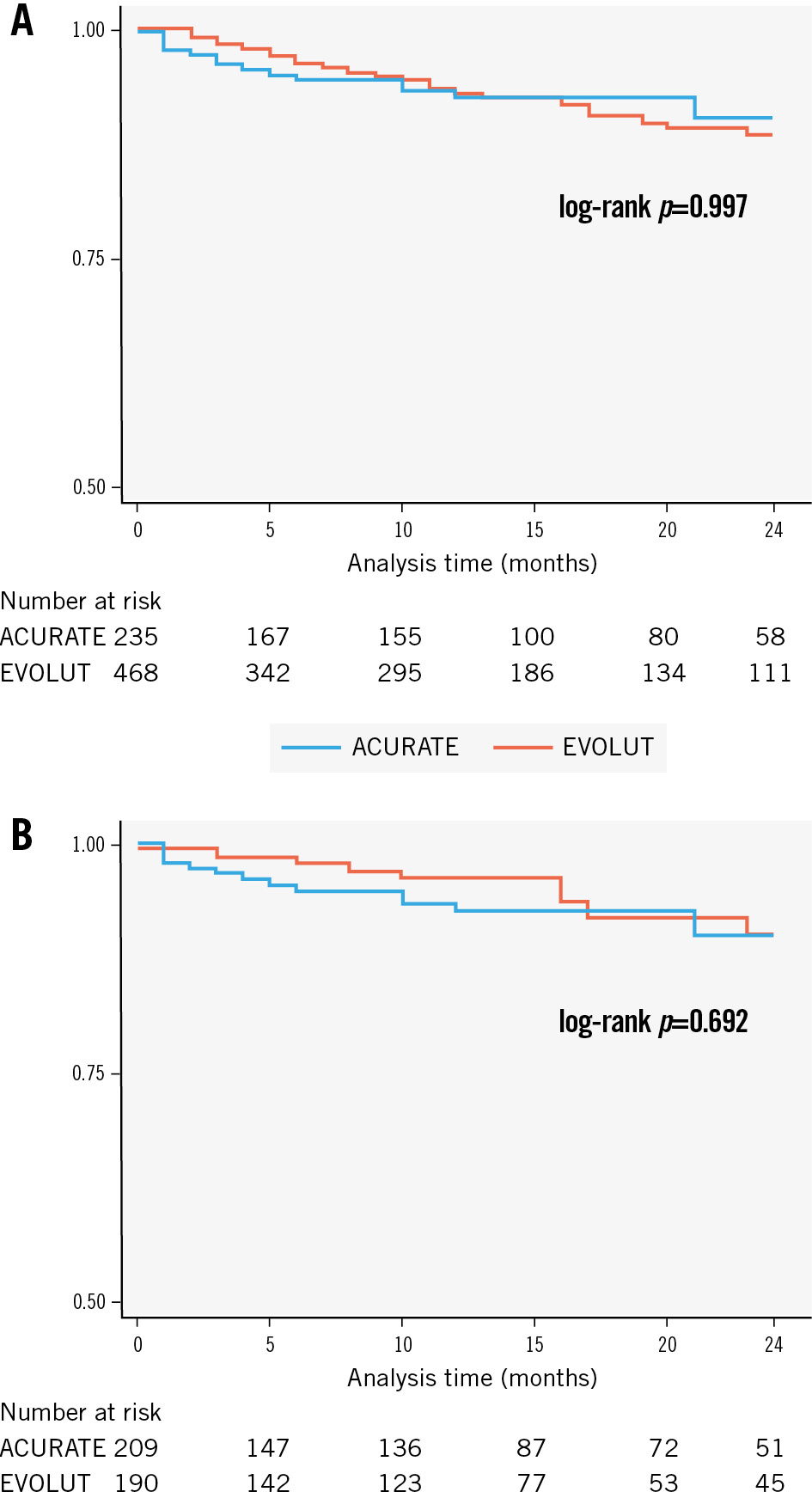
Figure 5. Kaplan-Meier survival curves. Survival curves are stratified according to the type of transcatheter prosthesis. At 24 months, there was no difference in all-cause mortality between ACURATE and Evolut THVs both in the unmatched (A) and matched (B) cohorts. ACURATE: ACURATE neo/neo2 group; EVOLUT: Evolut R/PRO/PRO+ group
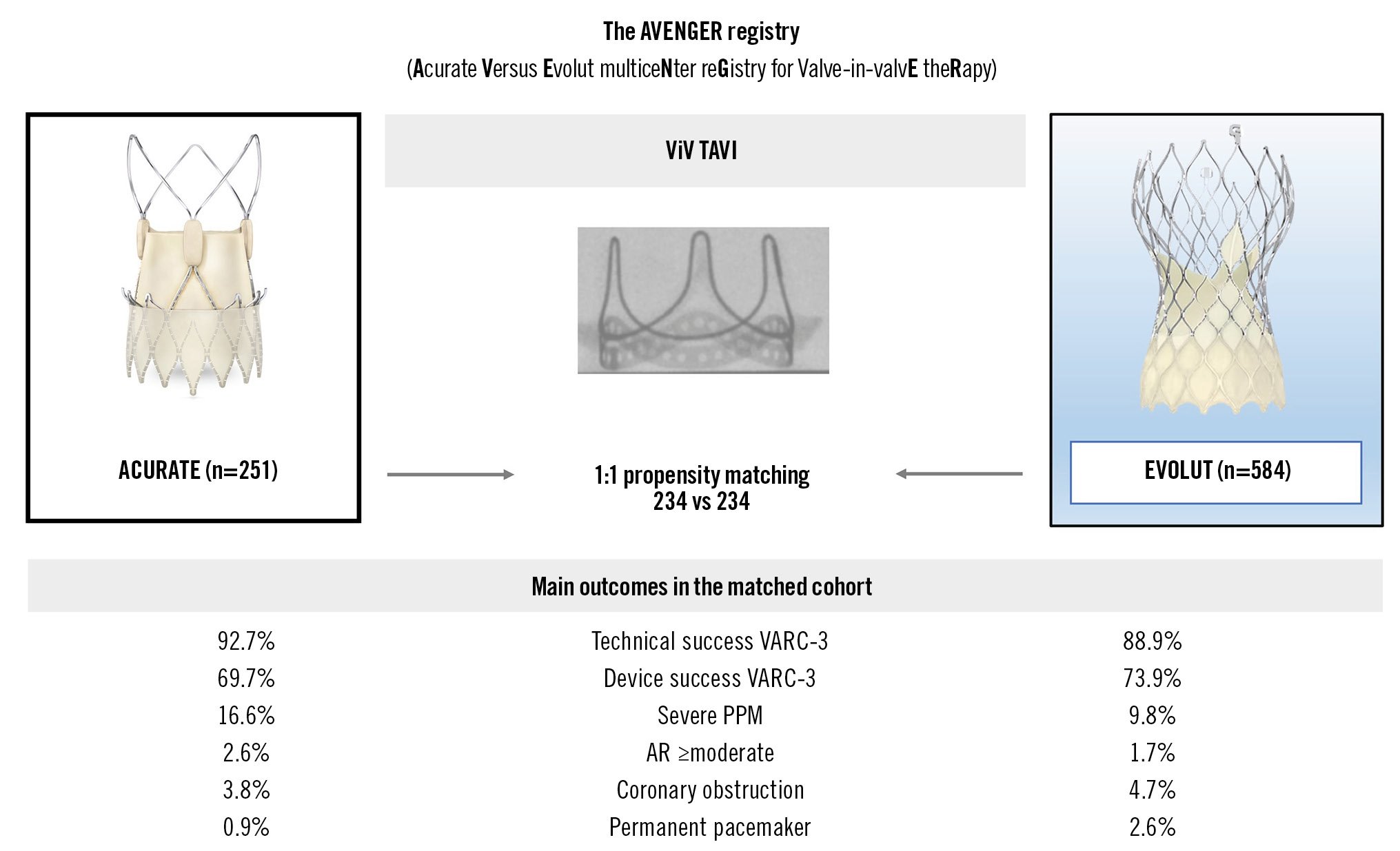
Central illustration. Main outcomes comparing ACURATE versus Evolut for ViV. The Central illustration provides an overview of the main outcomes after propensity score matching. Technical success, device success, rates of severe prosthesis-patient mismatch (PPM), aortic regurgitation (AR) ≥moderate, coronary obstruction, and permanent pacemaker implantation were similar between the ACURATE and EVOLUT groups. ACURATE: ACURATE neo/neo2 group; EVOLUT: Evolut R/PRO/PRO+ group; VARC: Valve Academic Research Consortium; ViV: valve-in-valve
Discussion
MAIN FINDINGS
In the largest comparative case series of ViV TAVI using SE devices to date, procedural outcomes were similar for both the ACURATE and the Evolut platforms. The need for PPI overall was rare. Survival curves showed no differences up to 24 months.
The only study comparing two different SE THV systems for ViV is from the International Valve-in-Valve Registry (Evolut CoreValve n=108 vs Portico [Abbott] n=54). It showed that there were no differences regarding procedural success, but postprocedural haemodynamic outcomes were more favourable and the rate of AR ≥moderate was lower (4.2% vs 13.7%; p=0.04) in the CoreValve group16.
Data on ViV using the ACURATE platform are scarce and limited to one multicentre case series using the first-generation ACURATE neo5. This early experience demonstrated the feasibility of ACURATE neo for ViV in 85 patients and further illuminated the impact of implantation height on haemodynamic outcomes as well as valve performance during midterm follow-up. Despite overall low rates of bioprosthetic valve failure, two cases of leaflet tears occurred during intermediate follow-up, which were not replicated in bench tests under various hydrodynamic positions5.
PROCEDURAL OUTCOMES
It should be taken into account that most prior studies were assessed according to VARC-2 criteria, whereas the present analysis was based on the novel VARC-3 criteria that uses different definitions of procedural success. Nonetheless, in a meta-analysis, device success (VARC-2) of ViV was 93.4% and is thus in the range of the technical success according to VARC-3 we found for both platforms (ACURATE 91.2%; Evolut 89.2%)3. Device success as defined by VARC-3 additionally incorporates 30-day mortality, parameters of valve function, and major vascular or structural complications, and therefore was much lower for both platforms. However, procedural success for ViV, as described by Landes et al, that included criteria of valve function was in a similar range with 62.4%17. The rate of ≥moderate AR was similar between the two groups in our cohort (ACURATE 2.4% vs EVOLUT 3.0%) and hence within the range of previous data351618.
Notwithstanding higher rates of predilatation and post-dilatation, the procedural duration and fluoroscopy times were shorter in the ACURATE group, and the amount of contrast agent used was lower. Despite the resheathability and repositionability of the Evolut platform, these findings indicate that the implantation of the ACURATE system is relatively straightforward.
SUBGROUP ANALYSES
The subgroup analyses indicate a possible advantage for the Evolut platform among patients with a true ID ≤19 mm in terms of device success, whereas the ACURATE platform showed higher technical and device success rates in failed SAVs with a true ID >19 mm. Device success was likely driven by increased gradients as shown in Supplementary Table 3, which was numerically more common in the ACURATE group. Hence, it may be assumed that in small SAVs, the expansion of the ACURATE prosthesis may be constrained because of the upper crown, especially in the event of low implantation.
Another interesting observation is that predominant aortic regurgitation as a reason for SAV failure was advantageous in the ACURATE platform, presumably due to less leaflet calcification.
HAEMODYNAMIC PERFORMANCE
There is an increasing awareness of the importance of haemodynamic performance following valve intervention19. In the present comparison, postprocedural mean gradients and the proportion of severe PPM were similar between the groups. Postprocedural aortic valve area was significantly greater in the EVOLUT group, but given the similar values of the median, a clinical relevance of this difference is unlikely, and values in the EVOLUT group were only based on 43% of cases (vs 74.5% in the ACURATE group) which questions the validity of this specific comparison. The measured indexed EOA was larger than expected for the EVOLUT group, whereas it was smaller than expected for the ACURATE group, which may indicate better haemodynamic characteristics of the Evolut platform, particularly in small SAVs. However, the clinical relevance is questionable, as the differences were small and did not reach statistical significance.
The haemodynamic outcomes of the Evolut platform in the present study are consistent with most previous reports. Data from the VIVID registry showed a larger EOA (1.67 cm2 vs 1.31 cm2; p=0.001) and lower mean gradients (14±7.5 mmHg vs 17±7.5 mmHg; p=0.02) in the CoreValve group. Rodes-Cabau et al compared balloon-expandable THV (n=46) with the supra-annular SE CoreValve THV (n=52) in the setting of ViV in small SAVs20. They reported lower mean transvalvular gradients (15±8 mmHg vs 23±8 mmHg; p<0.001) and a trend for less severe PPM for SE devices. In a meta-analysis, the average mean transvalvular gradient was similar between SE and BE devices (14.7 mmHg vs 15.6 mmHg), but the mean EOA was larger for SE devices (1.5 cm2 vs 1.2 cm2; p<0.001)3. A very recent comparison between several THV platforms for ViV showed lower gradients when using the Allegra (New Valve Technology) THV in comparison with the CoreValve THV (14.9±5.0 mmHg vs 18.7±7.5 mmHg; p=0.0295)21.
PROCEDURAL ASPECTS AFFECTING HAEMODYNAMICS
The favourable effect of post-dilatation and bioprosthetic valve fracture (BVF) on haemodynamic function has been demonstrated via hydrodynamic testing and in a clinical setting22. In the present study, post-dilatation was more common in the ACURATE group, which may be attributed to the lower radial force and the different principle of deployment and anchoring of the ACURATE platform. The proportion of attempted BVF was similar but slightly differed regarding the timing and success, as specified in Table 2. While the timing was at the discretion of the individual operator and may have varied according to individual standards at each centre, the success rate may not necessarily be related to the THV platform but rather to the characteristics of the failed surgical prosthesis. The impact of the implantation depth on haemodynamics after ViV was reported by Simonato et al23. For the Evolut platform, the optimal implantation depth has been defined to be between 0 and 5 mm to achieve favourable haemodynamics, but such a specific recommendation does not exist for the ACURATE valve. Even though in the present study the implantation depth in the ACURATE group was smaller, a direct comparison with the Evolut platform may not be appropriate given the above-mentioned differences regarding deployment and anchoring. However, previous bench tests have suggested that when the upper crown of the ACURATE valve is positioned above the stent posts of the failed SAV, this is associated with lower transvalvular gradients5.
CORONARY OBSTRUCTION
Reported rates of CO for aortic ViV procedures range between 0.6% and 3.5%, whereas in a meta-analysis the rate was reported to be 8.2%13. In the present analysis, CO rates were slightly higher, as previously described, which may be explained by the CO definition that included the deployment of the coronary stent despite sufficient coronary flow at the discretion of the operator. Hence, along with the fact that all COs occurred in the second half of the study period, this indicates that the threshold for “prophylactic stenting” may have become lower over time. In addition, the proportion of stented bioprostheses with externally mounted leaflets was relatively high in the present cohort.
When stratified according to the CO risk, the incidence of CO was similar between the two groups (Figure 4).
PERMANENT PACEMAKER IMPLANTATION
For the ACURATE platform, there is only one previous study that showed a low PPI rate of 1.4% in a series of 72 ViV cases (n=1), whereas for the Evolut platform, there is a bulk of evidence, with PPI rates ranging from 6.7% to 12.0%31618. The largest existing study on PPI following ViV is by Alperi et al, showing an average PPI rate of 6.4%, with lower rates for new-generation valves and a possible impact on mortality at intermediate follow-up24. In the present analysis, the rate of PPI was very low overall and almost negligible for the ACURATE platform, which may be attributed to the lower radial force of the ACURATE stent frame and its specific distribution of the outward force25.
In addition, implantation depths were smaller in the ACURATE than in the EVOLUT group. A detailed review of the two PPI cases in the ACURATE group showed that pre-existing conduction disturbances were present at baseline in both cases.
FUTURE PERSPECTIVES
It is key to accumulate data and identify the best possible treatment for patients requiring ViV, in particular regarding haemodynamic factors and the risk of coronary obstruction but also with respect to coronary access. Even though the latter was not specifically assessed in the present study, previous evidence suggests that coronary cannulation might be more difficult after implantation of prostheses with a long stent frame with relatively small struts, such as the Evolut family26. This has been demonstrated in a bench study using 3D-printed models of ViV cases for coronary cannulation. However, the clinical evidence needs to be studied in a prospective setting.
Limitations
The results of the present study need to be interpreted in the light of several limitations. It is a retrospective analysis without prospective data collection and prespecified endpoints. Insights into haemodynamic performance after ViV procedures with both platforms are limited to postprocedural intrahospital results with a lack of long-term haemodynamic outcomes. Echocardiography, MDCT, and fluoroscopic images were not evaluated by a core laboratory. Although clinical events were categorised according to standardised VARC-3 definitions, events were not adjudicated independently. The presence of pre-existing PPM was estimated by calculation of the projected PPM derived from reference values of the normal EOA.
Conclusions
ViV TAVI using either ACURATE or EVOLUT THVs showed similar procedural outcomes.
However, a true ID >19 mm was associated with higher device success among ACURATE recipients, whereas in patients with true ID ≤19 mm device success was higher when using Evolut.
Impact on daily practice
ViV TAVI for failed surgical bioprostheses shows favourable procedural outcomes for both the Evolut and ACURATE platforms with high rates of technical success and excellent haemodynamics. Overall, permanent pacemaker implantation is very uncommon and almost negligible when using the ACURATE valve. Randomised comparisons of available THV platforms with longer-term follow-up are warranted to provide evidence for optimal patient-tailored treatment.
Acknowledgements
We thank Elizabeth Martinson, PhD, of the KHFI Editorial Office, for her editorial assistance.
Conflict of interest statement
W. Kim reports personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Meril Life Sciences, Shockwave Medical, and HiD Imaging. M. Seiffert received speaker or advisory fees from Abbott, Abiomed, Amgen, AstraZeneca, Boston Scientific, Bristol-Myers Squibb, Daichii Sankyo, Edwards Lifesciences, Inari Medical, Medtronic, Pfizer, Shockwave Medical, and Siemens Healthineers; and a research grant from Boston Scientific - all unrelated to the submitted work. A. Rück has received grants from Boston Scientific; and personal fees from Boston Scientific and Edwards Lifesciences. D. Leistner reports personal fees and non-financial support from Abbott; personal fees from Boston Scientific; and grants from DZHK (German Center for Cardiovascular Research). H. Dreger is on the advisory board and received speaker fees from Abbott and Edwards Lifesciences; and is a proctor for and received research funding from Abbott. M. Adam reports personal fees/speaker honoraria from Abbott, Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic. H Möllmann reports proctor fees and/or speaker honoraria from Abbott, Biotronik, Edwards Lifesciences, Medtronic, SMT, and Boston Scientific. J. Blumenstein reports proctor fees from Boston Scientific. A. Holzamer reports proctoring for Boston Scientific. M. Barbanti is a consultant for Medtronic, Edwards Lifesciences, and Boston Scientific. C. Tamburino is a consultant for Medtronic. F. Castriota is a proctor for Boston Scientific, Medtronic, Abbott, and Terumo. R. Nerla is a proctor for Medtronic. C. Frerker received travel support and lecture honoraria from Medtronic and Boston Scientific. T. Schmidt received travel support and lecture honoraria from Medtronic and Boston Scientific. A. Wolf is a proctor for Medtronic, Boston Scientific, and Edwards Lifesciences. S. Toggweiler has served as a consultant for Medtronic, Boston Scientific, Edwards Lifesciences, Medira, Shockwave Medical, Teleflex, AtHeart Medical, Veosource, Polares Medical, and Biosensors; has served as a proctor for Medtronic, Boston Scientific, Edwards Lifesciences, Biosensors, and Abbott; has received institutional research grants from Boston Scientific, Biosensors, Fumedica, and Novartis; and holds equity in Hi-D Imaging. A. Mangieri received speaker honoraria from Boston Scientific and Abbott; received fees and speaker honoraria from Concept Medical; has received institutional grants from Boston Scientific, Abbott, and Kardia; and serves as proctor for Kardia. G. Kaleschke received speaker fees and travel grants from Edwards Lifesciences. E. Charitos reports proctor fees from Boston Scientific. M. Joner reports institutional grant support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and Infraredx; consulting fees from Biotronik, TriCares, Veryan, and Shockwave Medical; speaker fees from Abbott, AstraZeneca, Biotronik, Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, ReCor Medical, and Shockwave Medical; reports participation on the steering committees of Biotronik and Edwards Lifesciences; travel support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and SIS Medical. M. Vanhaverbeke is a consultant for Boston Scientific. M. Renker received proctor fees from Boston Scientific. T. Rheude received lecture fees from Abbott, AstraZeneca, SIS Medical AG and Translumina. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

