Abstract
Aims: In patients undergoing transcatheter aortic valve implantation (TAVI), the high prevalence of peripheral artery disease (PAD) limits femoral access and increases vascular complications that are associated with mortality and morbidity. Our study assessed the ability of a balloon-expandable large-bore vascular sheath to increase access-site availability and to reduce vascular complications.
Methods and results: Among 257 patients from two centres, 43 patients underwent transfemoral TAVI with the use of the SoloPath balloon-expandable sheath due to complex iliofemoral access anatomy. Propensity score matching (2:1) was performed except for the sheath to femoral artery ratio (SFAR). Compared to standard sheath patients, we found no significant difference in 30-day and one-year mortality (SoloPath vs. standard sheath, 9.3% vs. 3.5%; p=0.2, and 18.6% vs. 23.3%; p=0.7), major vascular complications (9.3% vs. 4.7%; p=0.3), and major bleeding (9.3% vs. 10.5%; p=0.5) in the cohort with the balloon-expandable sheath.
Conclusions: The use of a balloon-expandable large-bore sheath in patients with a high risk for vascular complications due to complex access-site anatomy proved to be feasible and safe. However, circumferential calcifications and sheath-to-artery ratios account for vascular access complications even in patients treated with the balloon-expandable sheath.
Abbreviations
AVA: aortic valve area
BMI: body mass index
CABG: coronary artery bypass graft
CFA: common femoral artery
CIA: common iliac artery
COPD: chronic obstructive pulmonary disease
EIA: external iliac artery
LES: logistic EuroSCORE
LVEF: left ventricular ejection fraction
MI: myocardial infarction
MSCT: multislice computed tomography
PAD: peripheral artery disease
PAH: pulmonary arterial hypertension
PCI: percutaneous coronary intervention
PSM: propensity score matching
SEIAR: sheath to external iliac artery ratio
SFAR: sheath to femoral artery ratio
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
Introduction
In transcatheter aortic valve implantation (TAVI), transvascular access via the femoral arteries is the preferred route. However, the prevalence of peripheral artery disease (PAD) in these patients ranges between 19.2% and 43%1,2 and may limit transfemoral access. PAD has been found to be an individual predictor of postoperative mortality3 and is associated with increased rates of vascular complications and poor outcome4,5.
Recent research has focused on the identification of predictors for vascular complications and has identified centre experience, sheath to femoral artery ratio (SFAR, >1.05), circumferential calcification, and female gender as important predictors for iliofemoral complications4.
To increase manoeuvrability in scenarios of complex iliac vascular anatomy and to minimise frictional forces caused by large-bore devices, the use of inflatable vascular sheaths has been evaluated6. Recent studies have suggested that these sheaths bear the potential to increase the availability of femoral access in patients with access-limiting PAD7 and may reduce vascular complications by reducing longitudinal insertion forces.
Our study assessed the ability of a balloon-expandable large-bore vascular sheath to increase access-site availability and to reduce vascular complications in an unselected two-centre TAVI cohort.
Methods
TECHNICAL SPECIFICATIONS
The SoloPath® balloon-expandable transfemoral access system (Terumo Medical Corporation, Irvine, CA, USA) is a polymer vascular sheath. The sheath is mounted on a central non-compliant expander balloon. The SoloPath sheath is inserted percutaneously over a stiff guidewire (e.g., Amplatz Super Stiff™; Boston Scientific, Marlborough, MA, USA, or Extra Stiff; Cook Medical, Bloomington, IN, USA). The SoloPath 19 Fr sheath with an unexpanded external diameter of 4.3 mm was used in all patients. These sheaths have an expanded sheath outer diameter of 7.3 mm. Standard sheaths used: Check-Flo® sheath (Cook Medical), outer diameter 7.2 mm, or Ultimum™ sheath (St. Jude Medical, St. Paul, MN, USA), outer diameter 6.8 mm8. The use of either standard sheath was left to the discretion of the operator.
STUDY POPULATION
Between 2010 and 2013, 257 patients underwent transfemoral TAVI with a Medtronic CoreValve® transcatheter heart valve (Medtronic, Minneapolis, MN, USA) at two institutions. Forty-three patients were treated using a SoloPath balloon-expandable sheath (35 patients at the University Hospital Bonn, and eight patients at the Krankenhaus der Barmherzigen Brüder, Trier, Germany). The SoloPath sheath was used mainly due to limiting access-vessel diameter, complex vascular anatomy (e.g., kinking), and/or calcification. The individual indication was categorised into five variables: 1) common femoral artery (CFA) diameter <6.5 mm; 2) tortuosity >2; 3) calcification >2; 4) a combination of tortuosity ≥2 and calcification ≥2 and access vessel diameter ≤7 mm; as well as 5) clinical decision/physician’s discretion. Vessel tortuosity and calcification were assessed by multislice computed tomography angiography images with use of a post-processing imaging software (3mensio Valves™; 3mensio Medical Imaging BV, Bilthoven, The Netherlands) by two blinded, independent physicians (A. Sedaghat and C. von Dobbeler). Tortuosity was graded from zero to four (no tortuosity → tortuosity ≥90°) and from zero to three for calcification (no calcification → severe calcification)9. Minor and major vascular complications, major bleeding events according to the updated VARC-2 criteria, and overall mortality were determined10.
STUDY ENDPOINTS
The primary study endpoint was a composite consisting of 30-day mortality, major vascular complications, and major bleeding according to VARC-2 definitions. Secondary endpoints included mortality at 30 days and one year, vascular complications and access-related complications (VARC-2 criteria)10. The need for red blood cell (RBC) transfusion was assessed. Major bleeding was defined according to the updated VARC-2 criteria and according to the Bleeding Academic Research Consortium definitions11.
PROCEDURAL DETAILS
All patients were discussed within the local interdisciplinary Heart Team. In this study, only patients undergoing a transfemoral TAVI were included. All patients agreed to participate in the local TAVI registry and gave written informed consent. The ethical committee of the University Hospital Bonn approved the registry. Generally, transfemoral approach is the first-line access at our two centres. Second-line approaches include transapical, transaortic, and transsubclavian depending on comorbidities and/or contraindications. Indications for alternative access sites are discussed within the Heart Team and decisions as to whether to choose a transfemoral or alternative access are based on imaging modalities (multislice computed tomography, duplex sonography) and comorbidities (previous chest surgery, radiation, severe lung disease, severe calcification, severe aortic disease and others).
TAVI was performed under conscious sedation in all patients. After percutaneous puncture of the common femoral artery proximal to the bifurcation, a closure system (Prostar® XL vascular closure system; Abbott Vascular Devices, Redwood City, CA, USA) was introduced for pre-closure of the access vessel. The stenotic aortic valve was crossed and, after changing to a stiff wire, a standard 18 Fr large-bore sheath (Check-Flo sheath [Cook Medical], outer diameter 7.2 mm, or Ultimum sheath [St. Jude Medical], outer diameter 6.8 mm, or the SoloPath sheath, deflated outer diameter 4.3 mm) was advanced under fluoroscopic control. Correct placement of the device was ensured by tracking of the radiopaque tip. When using the SoloPath sheath, the inserted sheath first had to be expanded by balloon inflation through injection of a 20 ml saline contrast mix for 60 seconds with 20 atm, resulting in an outer diameter of 7.3 mm. The inner balloon was then removed and the implantation of the self-expanding transcatheter heart valve was performed as previously described12. For safety reasons, a pigtail from the contralateral side was placed in crossover technique into the iliofemoral vessels before removing the sheath. In all patients, the Prostar XL vascular closure system was used for closing the puncture site. The integrity of the vessel and the degree of extravasation at the puncture site were assured using angiography. Access-site complications were treated by stent implantation at the physician’s discretion13. Postoperatively, the patients were transferred to the intensive care unit and monitored for at least 48 hrs. Dual antiplatelet therapy with 500 mg aspirin and 300 mg clopidogrel (the day before the procedure) was given as the standard peri-interventional antithrombotic regimen. Intravenous heparin was administered in a dose of 70 IU/kg, with additional boluses to maintain an ACT of >250 seconds during the procedure.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation. For statistical evaluation, SPSS Version 20.0.0 (IBM Corp., Armonk NY, USA) was used. Student’s t-test or Mann-Whitney test was applied depending on the given distribution. Categorical variables were compared with a chi-square test. We performed propensity score matching with the use of a logistic regression model in a 2:1 ratio. Covariates for matching analyses were chosen on the basis of significantly different baseline parameters. Matching was performed without replacement and with a caliper of 0.2 of the standard deviation of the logit of the propensity score (The R project for statistical computing, Vienna, Austria). The primary composite endpoint and mortality were evaluated by Kaplan-Meier estimates; p-values <0.05 were considered statistically significant.
Results
BASELINE CHARACTERISTICS
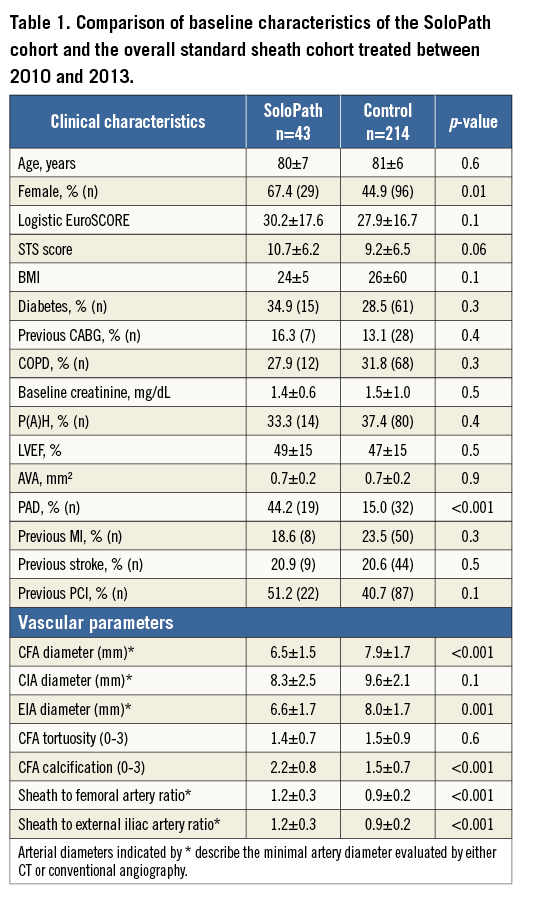
In our cohort of 257 patients, 43 patients underwent transfemoral TAVI with use of the SoloPath balloon-expandable sheath. Of these patients, 32.6% (14/43) were male with a mean age of 80±7 years and at high surgical risk (mean logistic EuroSCORE 30.2±17.6% and mean STS score 10.7±6.2%). Detailed analyses of baseline characteristics between groups are depicted in Table 1.
INDICATIONS FOR USING THE SOLOPATH SHEATH
In 38/43 (88.4%) patients, the SoloPath sheath was used as the first choice sheath. Among these, an access-vessel diameter smaller than 6.5 mm triggered the indication in 16/38 (42.1%) patients. Extensive access-vessel tortuosity was the indication in three patients (7.9%), and extensive femoral calcification in 18.4% (7/38). A combination of the above was present in nine patients (23.7%). The SoloPath sheath was used in three patients on the basis of a clinical decision (7.9%). In these three patients, CFA diameter and degree of femoral artery calcification were lower compared to the remaining SoloPath patients (CFA diameter: 8.2±1.3 mm vs. 6.4±1.4 mm, p=0.04; CFA calcification: 1.0±0 vs. 2.3±0.7, p<0.01). Femoral artery tortuosity was comparable with 1.3±0.6 vs. 1.4±0.7 (p=0.8), and was the main argument for using the SoloPath sheath in these patients.
In 11.6% (5/43), the SoloPath transfemoral access sheath was used as a bail-out strategy, caused by failure to advance a standard sheath. Secondary use of the SoloPath sheath was triggered by the inability to cross the iliofemoral junction in these patients. In comparison to patients with primary SoloPath use, these patients showed a tendency towards smaller access-vessel diameters and more extensive femoral artery calcification (CFA diameter: 6.2±1.6 mm vs. 6.6±1.5 mm, p=0.6; CFA calcification: 2.4±0.5 vs. 2.1±0.8, p=0.5).
PROPENSITY SCORE MATCHING
Propensity score matching (PSM) was performed in a 2:1 manner. The following variables were included on the basis of significantly different baseline characteristics between the SoloPath and the standard sheath cohort (Table 1): 1) gender, 2) STS score, 3) CFA diameter, 4) peripheral artery disease, and 5) degree of CFA calcification.
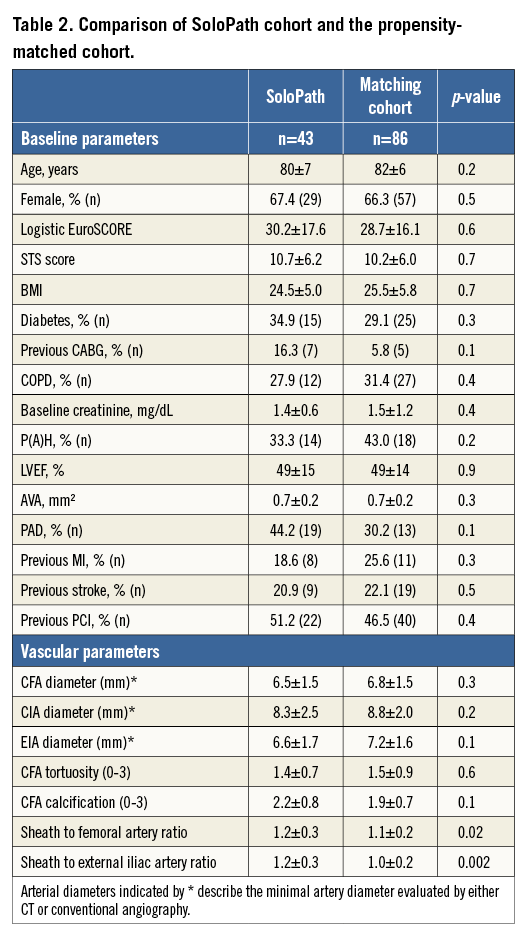
After matching for the covariates, no significant differences in baseline characteristics were observed between the SoloPath cohort (n=43) and the matched standard sheath cohort (n=86). However, trends towards an increased prevalence of previous cardiac surgery and peripheral artery disease remained. Matching for ratios of sheath-to-CFA and sheath-to-EIA could not be performed due to the lack of matching partners in the standard sheath cohort (Table 2).
PRIMARY ENDPOINT OUTCOMES
The primary composite endpoint (30-day mortality, and VARC-2 defined major vascular complications or major bleeding) occurred in 20.9% (9/43) in the SoloPath cohort and in 14.0% of the propensity score matched patients (p=0.2) (Figure 1).
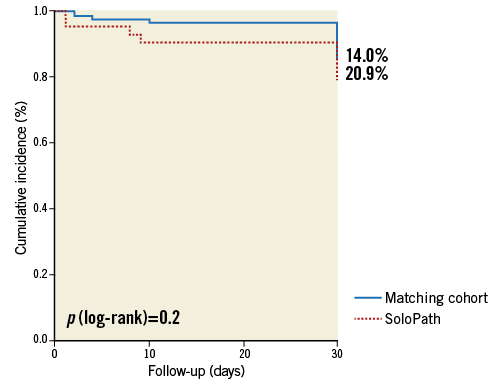
Figure 1. Kaplan-Meier estimates comparing the incidence of the combined primary endpoint in the SoloPath cohort to that in the propensity-matched cohort.
Mortality at 30 days and one year in the SoloPath cohort was 9.3% and 18.6%, respectively. In conventionally treated matched patients, 30-day and one-year mortality rates were 3.5% and 23.3%, respectively. No significant differences were observed for 30-day and one-year mortality (p=0.2 and p=0.7) (Figure 2, Figure 3).
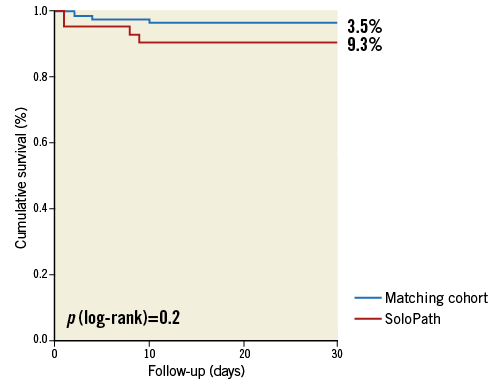
Figure 2. Kaplan-Meier estimates comparing 30-day mortality in the SoloPath cohort to that in the propensity-matched cohort.
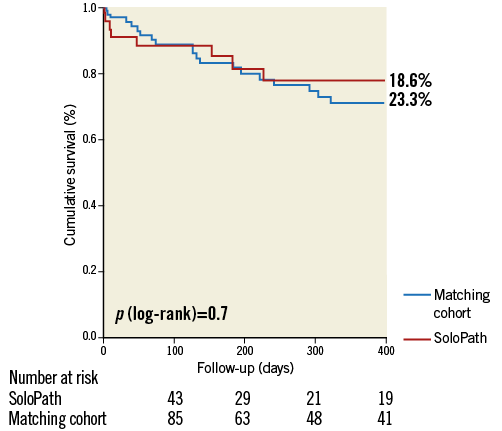
Figure 3. Kaplan-Meier estimates comparing 1-year mortality in the SoloPath cohort to that in the propensity-matched cohort.
VASCULAR OUTCOME
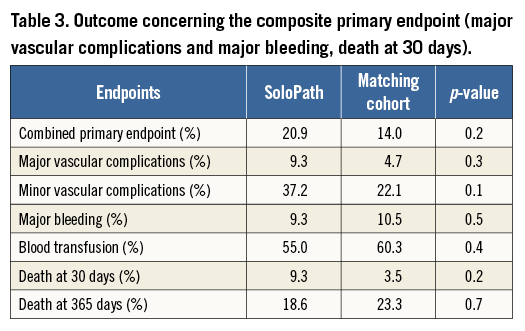
Non-significant differences in major vascular complications (4.7% in the matched control cohort vs. 9.3% in the SoloPath cohort, p=0.3) and major bleeding (10.5% in the matched control cohort vs. 9.3% in the SoloPath cohort, p=0.5) were evident. Minor vascular complications occurred in a comparable frequency in the standard and in the SoloPath cohort (22.1% vs. 37.2%, p=0.1). The need for blood transfusions was comparable between groups (55.0% vs. 57.7%; p=0.5) (Table 3). Vascular stent graft implantation was significantly higher in the SoloPath cohort with 30.2% vs. 12.8% in the propensity-matched cohort (p=0.02).
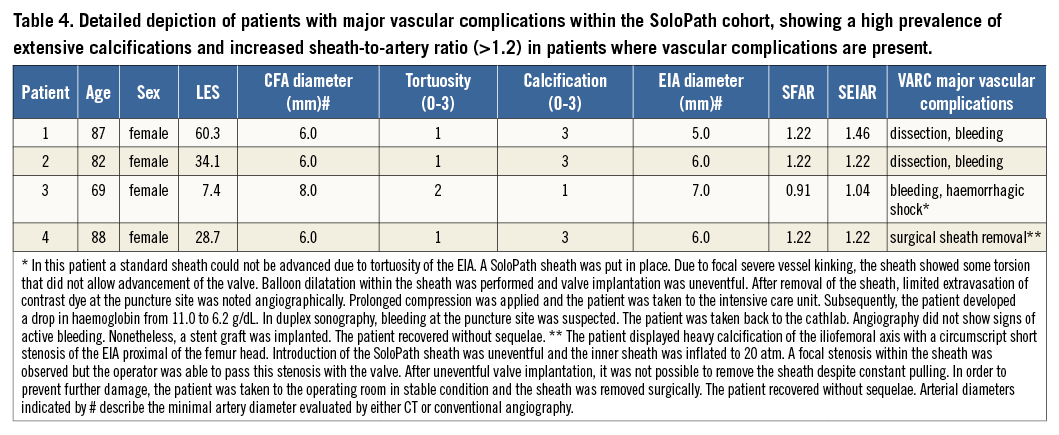
Detailed analysis of the major vascular complications in four patients who suffered from major vascular complications showed bleeding from the external iliac artery and femoral artery leading to haemorrhagic shock in two patients, and vessel dissection of the external iliac artery causing severe bleeding in one patient. Surgical intervention due to inability to remove the sheath was necessary in one case with severe circular calcification. All patients were female and three of the four had extensive femoral artery calcification. Detailed analysis of the access sites revealed increased sheath to femoral artery and sheath to external iliac artery ratios (Table 4).
Discussion
TAVI is an emerging therapy option in high-risk and non-operable patients with severe symptomatic aortic stenosis14. Although vascular complications in transfemoral TAVI have been reduced within recent years, they still represent a major limiting factor, especially in patients with complex vascular anatomies4. The availability of an inflatable sheath is appealing, as it may reduce vascular complications and extend the spectrum of patients who can be treated transfemorally.
We analysed 43 patients who were treated with the inflatable SoloPath sheath due to complicated access sites. The large majority of these patients showed severe vascular calcifications, small vessel diameters, and/or severe tortuosity that led the treating physician to refrain from using conventional 18 Fr large-bore sheaths. After propensity matching, the cohort treated with a SoloPath sheath showed comparable outcome and non-significant differences in both minor vascular complications and major vascular complications compared to the standard sheath cohort. Also, major bleeding and the need for blood transfusion were comparable.
Although inter-study comparison of vascular complications has only recently been optimised by the updated VARC definitions, interpretation of our results in the context of previously published data is paramount.
When looking at the incidence of major complications in our SoloPath cohort, complication rates are comparable to data published by Van Mieghem et al who reported the experience of five large European TAVI centres: in this study, major vascular complications occurred in 14.2% in a cohort of 969 all-comer patients15. Interestingly, the rate of major vascular complications in our SoloPath cohort was only 9.3%, despite a higher prevalence of PAD compared to that reported by Van Mieghem et al (44.2% vs. 16.0%).
In a similar analysis of 127 patients undergoing transfemoral TAVI with both Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) and Medtronic CoreValve prostheses, Hayashida et al reported a 17.3% incidence of VARC major vascular complications. In this study, the femoral artery mean diameter was as large as 8.17±1.14 mm and the mean sheath outer diameter was 8.10±0.82 mm, resulting in a SFAR of 0.994±0.155. When comparing both cohorts, it becomes apparent that femoral artery diameters were significantly smaller and SFAR was well above one in our cohort (1.2±0.3)16. In the light of this, an incidence of major vascular complications of 9.3%, in a cohort with difficult access sites, suggests the feasibility of a balloon-expandable sheath.
Taken together, despite smaller access-site vessels, unfavourable sheath to femoral artery ratio, more severe vascular calcification and tortuosity in our SoloPath cohort, the incidence of major vascular complications was comparable to published experiences. However, despite the overall good performance of the SoloPath sheath, the incidence of vascular complications was markedly higher in the SoloPath sheath cohort compared to our matched cohort. Rigorous analysis of our patients within the SoloPath cohort who experienced vascular complications revealed the following: three of four patients showed extensive access-vessel calcification, and the mean sheath to artery ratio for the external iliac artery was >1.2 in these patients. The predominant site of vascular injury was within the iliac artery close to the bifurcation (3/4 patients) and not within the puncture site, emphasising the importance of pre-interventional MSCT angiography with the use of the SoloPath sheath. In one case, we were not able to retrieve the sheath after the procedure. This was most likely due to severe circular calcifications of the external iliac artery. A sub-analysis of our cohort excluding patients with severe vascular calcifications (score >2) showed a remarkably low incidence of major vascular complications (3.7%), indicating an association of access-vessel calcification with SoloPath outcome. Careful patient selection including CT angiography and the analysis of calcification patterns might therefore be useful to minimise the risk of major vascular complications with the use of the SoloPath sheath.
In scenarios where access-limiting PAD or contraindications for transfemoral access are present, the transapical approach is currently the treatment of choice. Although numerous alternative vascular approaches have been published to date, transfemoral access remains the least invasive route17. Thus, the use of a dedicated balloon-expandable transfemoral access sheath is compelling, even in patients without access-limiting PAD: due to the decreased longitudinal stress, subsequent endothelial denudation of the iliofemoral axis and vascular access-site damage may be reduced with such a device. This aspect will gain special importance with a potential extension of TAVI to patients with intermediate risk, as late complications, e.g., vessel stenosis, are more likely to be evident in these patients.
As scientific data on the use of the balloon-expandable SoloPath sheath are scarce7, the results of our study shed some light on this dark area. However, randomised controlled studies will have to evaluate the role of balloon-expandable sheaths in PAD and non-PAD patients for TAVI procedures. Further technical advancements, such as the new-generation re-collapsible balloon-expandable sheath, will play a significant role in this context18.
Limitations
Major limitations of our analysis include the non-randomised character of the study associated with outstanding differences in terms of femoral-iliac anatomy despite propensity score matching (selection and therapy bias), the relatively small sample size (with most of the patients recruited from one centre), and the self-reported character of the study without a core lab.
Conclusions
The use of the SoloPath transfemoral access system in a propensity-matched cohort with a high risk for vascular complications proved to be feasible and safe compared to a standard cohort. However, the numerical incidence of vascular complications remained higher in patients with difficult access sites: this needs to be taken into consideration when choosing the optimal access site for TAVI. Further randomised studies are necessary to evaluate the role of balloon-expandable sheaths in the context of vascular injury and subsequent access-site complications in patients with and without PAD.
| Impact on daily practice The use of balloon-expandable access sheaths has been suggested for TAVI patients with limited femoral access, but the scientific evidence supporting the use of these sheaths is scarce. Our study indicates that the use of the SoloPath balloon-expandable sheath is safe and feasible in a transfemoral TAVI cohort with a high risk for vascular complications and results in outcome comparable with a propensity-matched standard sheath cohort. |
Conflict of interest statement
J.M. Sinning, G. Nickenig, E. Grube and N. Werner have received research support and speaker’s honoraria from Edwards Lifesciences and Medtronic. E.Grube and N. Werner are proctors for Medtronic. N. Werner has received research support and speaker’s honorarium from Terumo. The other authors have no conflicts of interest to declare.

