Introduction
Vascular access remains a major challenge in transfemoral transcatheter aortic valve implantation (TAVI) due to the need of large-bore introducer sheaths, ranging between 18 and 24 Fr inner diameter (outer diameter [OD] up to 28 Fr)1. The elderly patient population currently referred for TAVI often presents with advanced atherosclerosis of the iliofemoral axis. To accommodate the large-bore sheath required for TAVI, balloon angioplasty with or without stenting may thus become necessary before sheath insertion. Nevertheless, dissections, occlusions or ruptures of the iliofemoral arteries, which are encountered in 13% up to 32% of patients, significantly contribute to periprocedural morbidity and mortality2,3. In patients with diffusely calcified access vessels with diameters <7 mm, transfemoral TAVI may not be possible and TAVI may be performed transapically or via the axillary artery, which, however, increases procedural complexity and requires general anaesthesia4.
Technical specifications
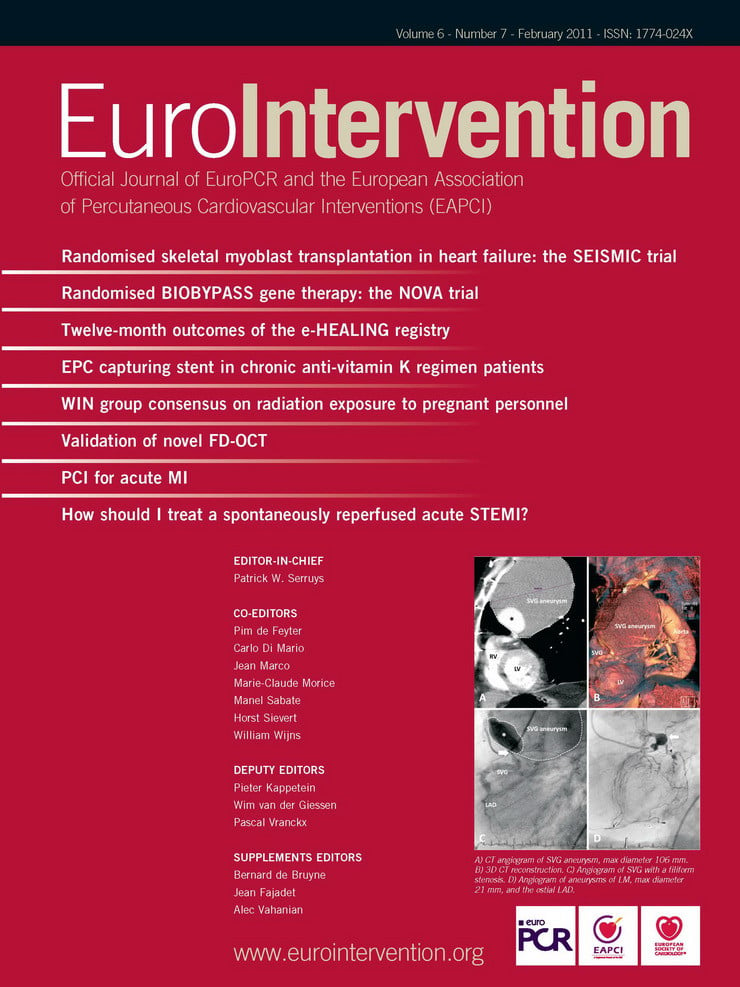
Here we discuss a novel balloon expandable vascular sheath to facilitate vascular access for transfemoral TAVI. This sheath (SoloPath™; Onset Medical Corporation, Irvine, CA, USA) (Figure 1) has an inner diameter of 19 Fr (OD 22 Fr) and is compatible with the 18 Fr Medtronic/CoreValve® delivery system (Medtronic, Inc., Minneapolis, MN, USA).
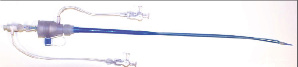
Figure 1. Photographic image of the 19 Fr balloon expandable sheath.
The distal section of the sheath is folded over a length of 25 cm with an outer diameter of approximately 13 Fr (Figure 2A), allowing facilitated vessel entry. After sheath insertion, a standard balloon inflation device is connected to the sheath-integrated balloon and the folded section of the sheath is expanded to its nominal 19 Fr diameter (Figure 2B).
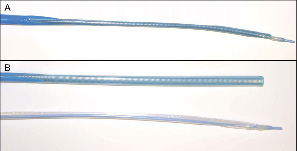
Figure 2. A) Magnification of the folded distal section of the expandable sheath with reduced diameter of approximately 13 Fr. B) After balloon dilatation the sheath has reached its nominal inner diameter of 19 Fr. The balloon was removed from the sheath (below).
Clinical experience
An 87-year-old male patient (logistic EuroSCORE 38.4%, STS score 9.2%) with severe aortic valve stenosis (mean gradient 53 mmHg, valve area 0.41 mm2) was scheduled for percutaneous implantation of a Medtronic/CoreValve prosthesis. Angiography of the iliac vessels showed a focal stenosis of the right external iliac artery with a minimal luminal diameter of 4 mm (Figure 3A).
Figure 3. A) Angiography of the right iliofemoral axis showing the focal stenosis of the right external iliac artery (arrow). B) Smooth advancement of the sheath over the iliac stenosis. C) Balloon expansion of the sheath to its nominal diameter after complete insertion into the vessel. D) Angiography after removal of the sheath showing the angioplasty-like result of the iliac stenosis.
The left iliac axis showed diffuse disease and was not deemed suitable for introduction of the large-bore access sheath. After puncture of the right common femoral artery, a single 6 Fr ProGlide™ device (Abbott Vascular Devices, Redwood City, CA, USA) was placed in a pre-close technique and exchanged for a 10 Fr sheath. A 6 Fr pigtail catheter was advanced from the contralateral side for intraprocedural angiography. The aortic valve was crossed using a 6 Fr AL 1 diagnostic catheter and exchanged over a 260 cm long 0.035’’ guide wire for a pigtail catheter. Then, a 260 cm Amplatz SuperStiff™ (Boston Scientific Corp., Natick, MA, USA) guidewire was introduced into the left ventricle with its spirally-shaped J-tip placed deep in the apex. The 10 Fr sheath was exchanged for the 19 Fr Solopath sheath. The folded distal section tracked smoothly through the subcutaneous track as well as through the iliac stenosis (Figure 3B). After complete insertion into the vessel, the sheath was expanded at 14 atm over one minute (Figure 3C). The sheath balloon was uneventfully removed. After valvuloplasty, a 29 mm Medtronic/CoreValve prosthesis was easily advanced through the sheath into the aortic annulus. Implantation resulted in a satisfactory valve position with complete elimination of the transvalvular gradient and only mild aortic regurgitation grade I (Figure 4).
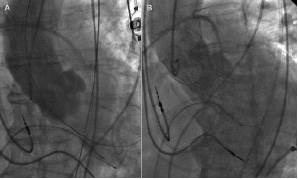
Figure 4. Angiography of the ascending aorta before (A) and after (B) transfemoral implantation of a 29 mm Medtronic/CoreValve prosthesis.
The delivery system was uneventfully removed through the sheath. Angiography showed an angioplasty-like result of the iliac stenosis by the sheath balloon without dissection (Figure 3D). After removal of the sheath, the pre-tied ProGlide knot was tied, resulting in complete haemostasis.
Conclusion
This report documents the successful use of a novel balloon expandable vascular access sheath for TAVI in a patient with significant atherosclerotic disease of the iliofemoral axis. Overall, we have now used the sheath successfully in a total of six patients, without any evidence of vascular injury or events of device malfunction. Further prospective evaluation of this promising approach is warranted.