Abstract
Aims: Prosthesis dislocation during transcatheter aortic valve implantation (TAVI) is a rare but important complication. There is scarce data on the performance of prosthesis in the aorta that have become dislocated from their intended anatomical position in the aortic annulus. We investigated the causes of dislocation during TAVI of the self-expanding CoreValve ReValving™ System (CRS) (Medtronic Inc., Minneapolis, MN, USA). This included midterm follow-up of patients experiencing this acute complication.
Methods and results: Among 176 consecutive patients undergoing TAVI with the CRS prosthesis, seven (3.9%) experienced acute valve dislocation. A comprehensive analysis of the mechanism of dislocation and clinical outcomes of patients experiencing this complication was performed. Based on the underlying mechanism, all cases of prosthesis displacement were classified into the following three groups: 1) accidental dislocation immediately after valve implantation (n=1; 14.3%); 2) dislocation during the snaring manoeuvre to reposition a low deployment of the CRS prosthesis (lower edge >10 mm) below the aortic annulus accompanied with haemodynamically significant regurgitation (n=4; 57.1%); 3) intentional dislocation performed with the snaring manoeuvre as a bailout in cases of coronary ostia impairment or severe prosthetic leak due to higher deployment for a suboptimal sealing of the device with valve calcifications (n=2; 28.6%). The majority of cases occurred during early experiences with the new Accutrak™ (Medtronic Inc., Minneapolis, MN, USA) delivery system. In six patients a second CRS was implanted in the appropriate position. The dislodged CRS functioned normally, without any evidence of structural deterioration, thrombosis or further distal migration and showed complete apposition against the aortic wall. No thromboembolic events were reported in any patient.
Conclusions: Dislocation of a CRS device can be managed effectively with the implantation of a second device in a standard fashion leaving the dislocated device safely in the aorta. Intentional repositioning of a CRS device in the aorta can be accomplished by experienced operators without any serious neurologic or vascular events in the post-procedure period or at midterm follow-up.
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as a viable alternative to surgical aortic valve replacement (AVR) for patients with severe symptomatic aortic stenosis (AS) considered as at unacceptable surgical risk1. The main technical impediment in the current generation of TAVI devices is the inability to totally retrieve and reposition the prosthesis in case of sub-optimal deployment. Prosthesis malposition during TAVI is not an uncommon finding2, and operator’s expertise is crucial in preventing device misplacement and to manage this complication whenever it occurs2. Prior reports suggest that in cases of implant failure, the self-expanding CoreValve ReValving™ System (CRS) (Medtronic Inc., Minneapolis, MN, USA) device can be mobilised and relocated in the aorta away from its original position in the aortic annulus. This bailout procedure has been used safely in most cases without any incremental risk for device malfunction, acute neurological events or excess periprocedural morbidity and mortality although a few cases of sequelae associated with the use of this technique have been reported in the literature.3-6
There is a paucity of data on the clinical and haemodynamic outcomes at midterm follow-up in patients who experience device dislocation while undergoing TAVI.
We report on the clinical outcomes of patients who experienced valve dislocation during TAVI with CRS at our institution.
Methods
Patients and procedure: From June 2007 until March 2011, 176 consecutive patients underwent TAVI using the third generation CRS prosthesis. Device characteristics and details of the procedure have been described previously7. All procedures were performed under fluoroscopic guidance by two experienced operators in a standard cardiac catheterisation laboratory with surgical back-up. Among the patients, seven (3.9%) experienced valve dislocation, and this subgroup formed the study population. A transthoracic echocardiogram (TTE) was performed after TAVI at discharge, and upon follow-up visit at 30 days, six months, 12 months and then annually, according to our institutional protocol. Frame integrity at follow-up was assessed with multislice computed tomography (MSCT).
Results
Overall, the snaring technique was used in 11 patients (6.2%). The reasons for using this bailout procedure and associated outcomes are shown in Figure 1. Salient clinical and echocardiographic characteristics of the study population are reported in Table 1. All patients (mean age 83±2 years, five female) were at high risk for surgical AVR (mean logistic EuroSCORE 23.2±10; mean STS Score 7.1±3). The transfemoral route was used in all procedures, and in all cases dislocation occurred immediately after CRS deployment. No cases of late dislocation were reported. A higher incidence of this complication occurred during initial experience with the new catheter delivery system Accutrak™ (Medtronic Inc.). In six out of seven procedures, a second CRS in the correct position was implanted during the same session.
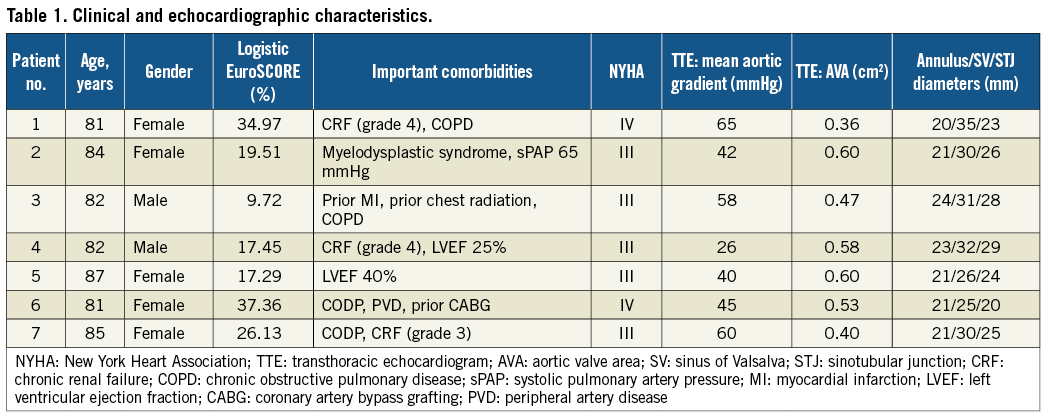
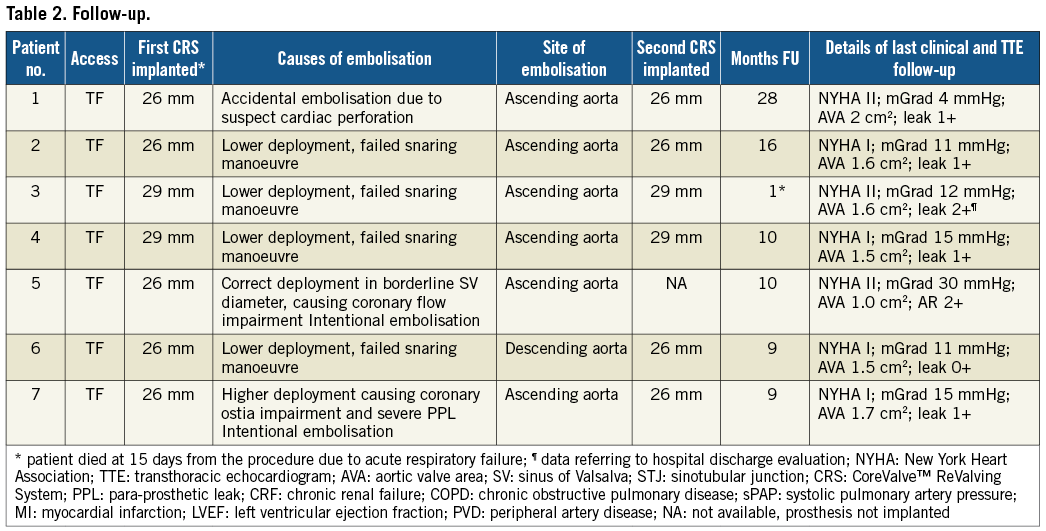
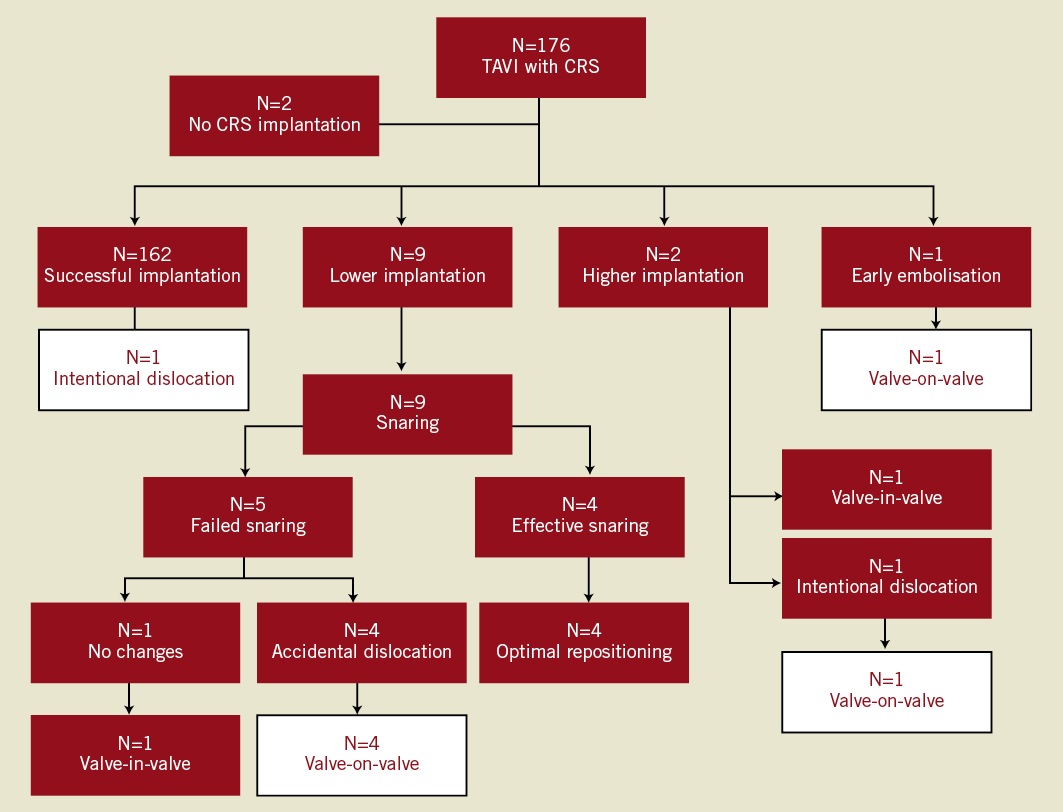
Figure 1. Flow chart of the management of prosthesis mal-positioning causing haemodynamic impairment. The white boxes indicate the patients forming the study population. CRS: CoreValve ReValving™ System.
CAUSES OF DISLOCATION
The reasons for prosthesis dislocation were classified into three groups (Table 2): 1) accidental dislocation immediately after valve implantation (n=1; 14.3%; case no.1); 2) dislocation during the snaring manoeuvre to reposition a low deployment of the CRS prosthesis (lower edge >10 mm) below the aortic annulus accompanied with haemodynamically significant regurgitation (n=4; 57.1%; case nos. 2, 3, 4 and 6); and 3) intentional dislocation performed with the snaring manoeuvre as a bailout in cases of coronary ostia impairment or severe prosthetic leak due to higher deployment for a suboptimal sealing of the device with valve calcifications (n=2; 28.6%; case nos. 5 and 7).
In case number 1, sinus bradycardia and arterial hypotension occurred during the release of the last part of the CRS prosthesis. Left ventricular perforation from the 0.035” stiff wire was suspected and the wire was immediately withdrawn. The prosthesis still anchored to the delivery catheter system (DCS) was retrieved and unintentionally deployed in the ascending aorta. After a few minutes, arterial pressure improved, the rhythm returned to sinus and TTE showed the presence of a minor pericardial effusion. It was decided to advance and place a second CRS, deployed in the correct position.
In case numbers 2, 3, 4 and 6, the lower edge of the CRS prosthesis was more than 10 mm below the level of the aortic annulus (Figure 2), causing haemodynamically significant paraprosthetic regurgitation evidenced by a low aortic diastolic pressure and an elevated left ventricular end diastolic pressure (LVEDP). The prostheses were well expanded and it was decided to reposition the valve using the “pull and stop snaring” technique2,8. The technique involved advancing a 30 mm GooseNeck™ catheter (Amplatz GooseNeck Microsnare™; EV3, Endovascular, Inc., Plymouth, MN, USA) through the 18 Fr introducer and after one of the delivery hooks of the implanted valve was secured with the snare loop, the device was gently pulled toward the aorta under continuous haemodynamic monitoring (Figure 3). These bailout procedures were however complicated with the accidental dislocation of the device in the ascending aorta. In one patient (case no.6) with two patent saphenous vein bypass grafts to the right coronary artery and a large obtuse marginal branch (Figure 4) the embolised prosthesis was positioned in the descending aorta using two GooseNeck snare catheters anchored to both delivery hooks to avoid trauma to the anastomosis or compromise bypass graft blood flow and then the prosthesis was dilated with a Nucleus 25/40 mm balloon (NuMed Inc, Hopkinton, NY, USA) to seal it to the aortic wall (Figure 5). Case numbers 3, 4 and 6 were performed during early experience with the new CRS delivery system Accutrak™ that incorporates an additional layer over the retractable delivery sheath that isolates the delivery sheath from the introducer and the vessel wall. In vitro, this new assembly reduces frictional forces thus providing increased stability during deployment and allows for an accurate final placement of the self-expanding prosthesis. The release technique with the new system is noticeably different than the older variant of the CRS system.
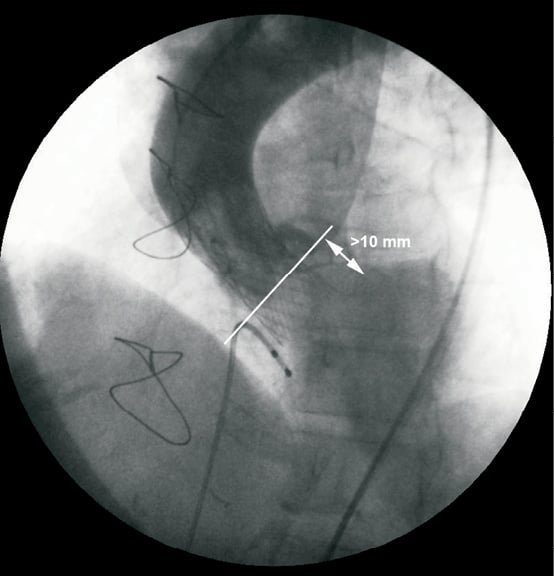
Figure 2. Too low implantation of a CoreValve™ prosthesis causing severe paraprosthetic regurgitation. The white line indicates the aortic annulus level.
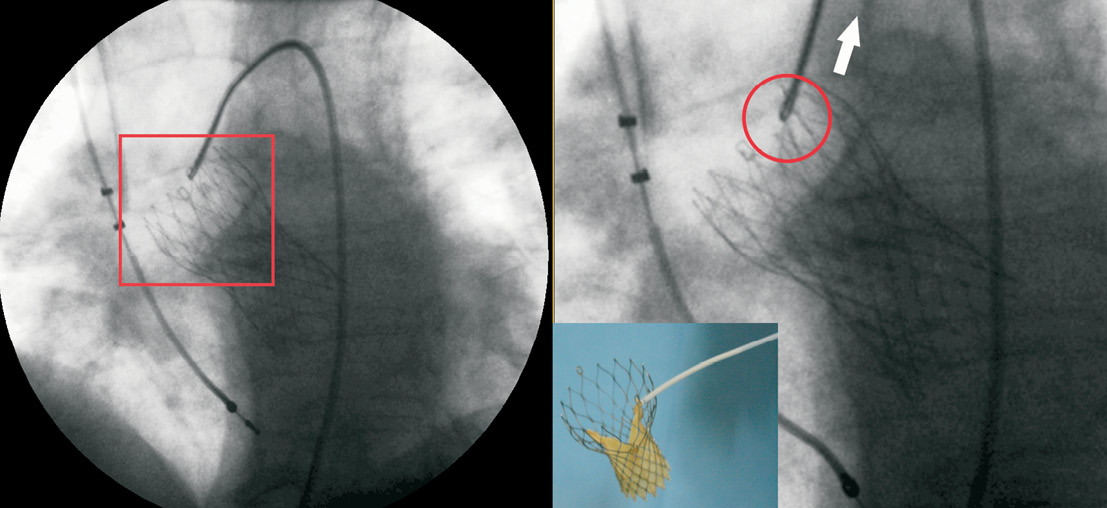
Figure 3. The prosthesis placed in a lower position is caught in one of the two hooks and gently, but firmly, pulled backward to the aorta.
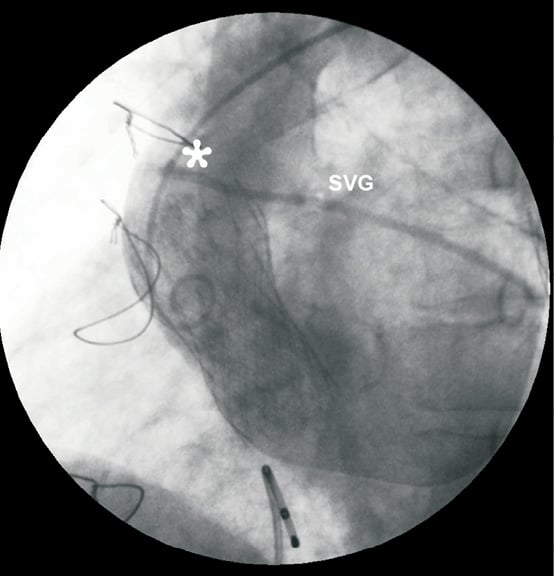
Figure 4. CoreValve™ prosthesis embolised in ascending aorta. The upper part of the frame is close to the aortic anastomosis (*) of a patent saphenous vein graft (SVG).
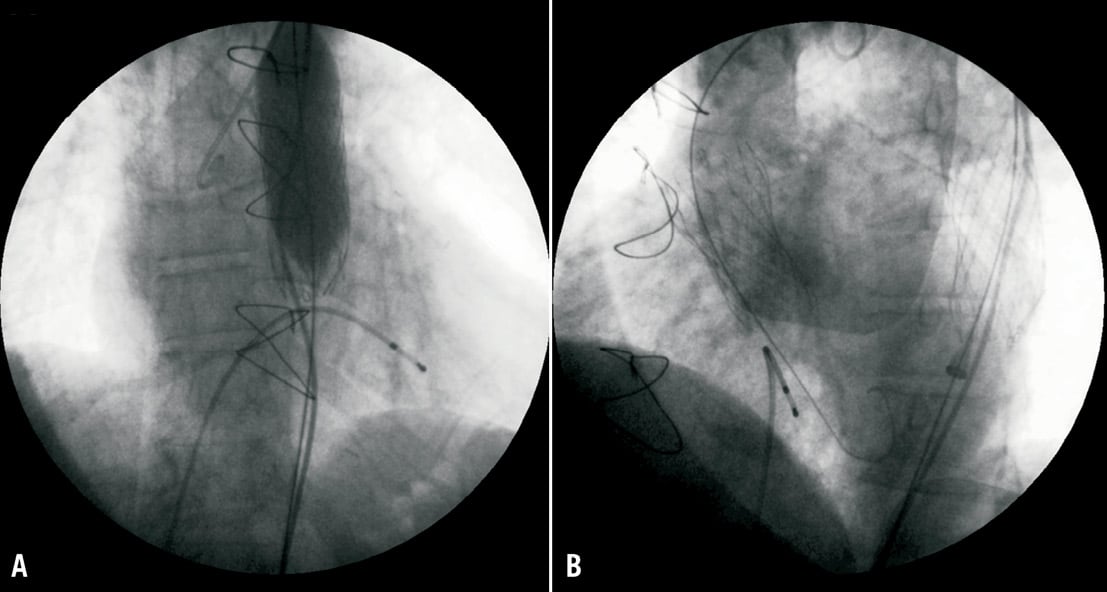
Figure 5. (A) The embolised prosthesis in descending aorta is crushed against the aortic wall with a standard valvuloplasty balloon. (B) The second CRS prosthesis correctly placed in its “anatomic” position.
In case numbers 5 and 7, valve dislocation using the snaring technique was performed intentionally as a bailout. In the first patient (case no.5) with severe cusp calcification (aortic annulus 21 mm, sinus of Valsalva 25.6 mm), a 26-mm CRS was advanced through the aortic arch and implanted appropriately inside the native valve. A post deployment aortogram showed subtotal obstruction of the left main coronary ostium by the calcified left coronary cusp, associated with severe hypotension and ST-segment elevation. A snare catheter was immediately advanced and the prosthesis was removed from its “anatomic” position and relocated in the ascending aorta (Figure 6). The coronary perfusion was restored immediately thereafter with resolution of ST-segment elevation and hypotension. In this case the TAVI procedure was aborted and the patient left the catheterisation laboratory after undergoing balloon aortic valvuloplasty (BAV) with a transvalvular peak-to-peak gradient of 25 mmHg and mild aortic regurgitation. In the second patient (case no.7), the valve was deployed too high leading to left main ostia impairment, as well as severe paraprosthetic regurgitation. The device was snared successfully with a GooseNeck catheter in the manner described above and retrieved in the ascending aorta. In this case, a second 26 mm CRS was advanced successfully through the aorta and the embolised prosthesis and released in the correct position with excellent procedural result.
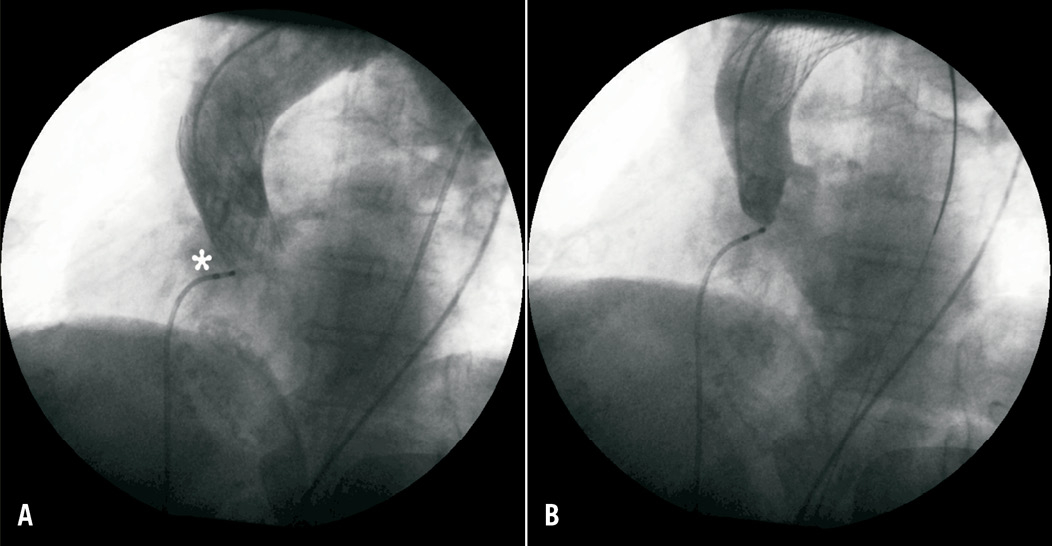
Figure 6. (A) CRS prosthesis correctly deployed inside the native valve, impairing right coronary ostia (*), due to small sinus of Valsalva dimension. (B) The same prosthesis placed in ascending aorta after intentional snaring.
In most cases (n=6) it was possible to navigate the second prosthesis through the first one and deploy it in the correct position. In one patient (case no.4), the second device could not be advanced beyond the transition between the ascending aorta and aortic arch through the outflow portion of the first device. In this particular case, a 25 mm GooseNeck snare was tightened around the delivery catheter just proximal to the site housing the collapsed valve, aligned with the aortic annulus, positioned across the native aortic valve and deployed in a standard fashion (Figure 7). In all cases no transvalvular gradient was recorded through the misplaced valves.
Most patients (n=6) were discharged without in-hospital complications. In case no.2, left femoral artery dissection was documented at the puncture site after arterial haemostasis was obtained with the Prostar™ XL 10 Fr system (Abbott Vascular, Abbott Park, IL, USA), necessitating the implantation of a covered self-expanding stent to cover the dissection site.
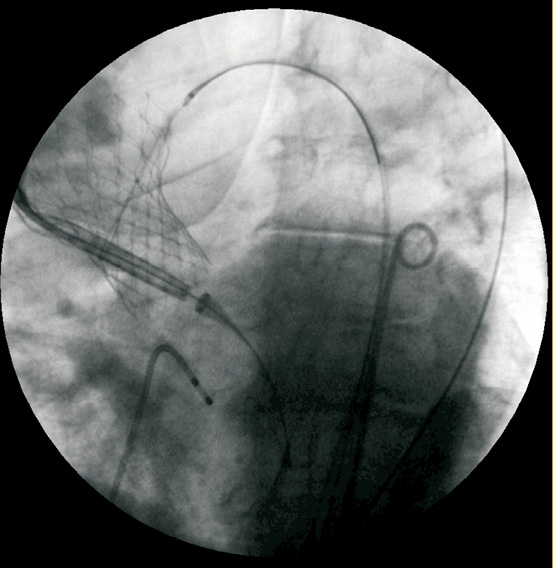
Figure 7. Second prosthesis advancing trough the first CoreValve™ placed in ascending aorta with the aid of the goose neck snare catheter.
FOLLOW-UP
Clinical and echocardiographic data at follow-up are displayed in Table 2. The median follow-up duration for the entire population was nine months (range one to 28 months). Patient no.3, who was discharged at day four after TAVI, and in New York Heart Association (NYHA) functional class II with normal TTE (left ventricular ejection fraction 45%; mean gradient 10 mmHg; aortic valve area 1.9 cm2; mild perivalvular leak), died at 15 days post-procedure due to acute respiratory failure. Beyond hospital discharge no other complications were reported in the study population. The misplaced CRS functioned normally, with no instances of structural deterioration, thrombosis or further distal migration, and showed complete apposition against the aortic wall. Moreover, no thromboembolic events were reported in any patient. For all deployed prosthesis, no signs of porcine tissue degeneration or frame fractures were reported. Patient no.7, who underwent only BAV was followed up to 10 months. She was in NYHA class II/III, and TTE showed mean transaortic gradient 30 mmHg and moderate aortic regurgitation.
Discussion
TAVI using stent-based bioprosthesis is being performed in several centres worldwide as an alternative to conventional surgery for patients affected by severe AS who are at unacceptable operative risk9-13. The procedure, using both self-expanding and balloon-expandable devices, is challenging and associated with a significant learning curve. Procedural success is closely linked to experience and is >90% in experienced centres9-13. It is almost impossible to reposition the current iterations of both the commercially available devices after initial deployment. Thus operators have to develop several percutaneous strategies to manage prosthesis malposition in the catheterisation laboratory (without emergent surgical bailout2-8).
The results of this study are in agreement with those recently reported by Gerckens and colleagues3. They assessed post-procedural and midterm outcome of nine patients (3.2% of the entire population), in which a second “in-series” CoreValve prosthesis was implanted during the same procedure, and showed that none of the 18 prostheses implanted had any sign of malfunction at follow-up. Among our study population we identified three distinct mechanisms for device dislocation: In case no.1, a suspected catastrophic complication (as detailed above) led to device dislocation4. Procedure numbers 2, 3, 4 and 6, where the CRS was deployed too low and the snaring manoeuvre caused device dislocation, merit further discussion. All cases except the first one (case no.2) were performed during early experience with the new Accutrak™ delivery system. In contrast with the previous third generation DCS for the CRS, which required the operator to pull on the system to prevent forward movement towards the ventricle during device release, the Accutrak™ delivery catheter has an additional stability layer that insulates the retractable delivery sheath from frictional forces from the 18 Fr introducer and the aortic wall, preventing significant forward movement of the CRS prosthesis during deployment. Although this improvement in device design might facilitate the prosthesis release, it makes the implanting technique quite different, and entails an additional learning curve. The valve-in-valve (ViV) technique has recently been demonstrated to be a useful bailout option to treat a malpositioned CRS (too low) with severe paraprosthetic regurgitation, in the absence of frame under-expansion. Use of the ViV technique as a bailout was associated with normal valve performance without any structural deterioration, coronary ostial obstruction, or cerebrovascular accidents at midterm follow-up14. In cases of low implantation, where there is no impairment of mitral leaflet coaptation due to the CRS frame, the valve-in-valve (ViV) technique might be a safer option as it avoids the potential complications associated with retrieval of the prosthesis along the ascending aorta. The durability of the ViV technique still needs to be confirmed at long-term follow-up in a large cohort of patients. The snaring manoeuvre is also a feasible approach for the problem of a low implant with haemodynamic deterioration2; however, the results of this manoeuvre remain unpredictable even in expert hands, and might result in the unseating and dislocation of the CRS device3. In this series, the ViV technique was used as first choice to treat paraprosthetic regurgitation only in one case of higher implantation and in another case of lower implantation after an unsuccessful snaring procedure.
Finally, cases nos. 5 and 7 highlight the importance of the snaring manoeuvre to manage unexpected and potentially fatal situations encountered during TAVI procedures. In both cases, the device deployment resulted in coronary ostia obstrucion due to calcified material from the aortic valve cusp. In the first patient with a 26 mm CRS implantation, the sinus of Valsalva proved to be too small (25.6 mm) to house the calcified cusps; therefore the patient left the catheterisation laboratory with only a BAV procedure. In the second patient with a high deployment of the CRS, the valve was snared out and placed in the ascending aorta; a second prosthesis was subsequently deployed correctly.
Data on Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) valve dislocation have also been reported recently. Tay and colleagues6 reported on seven cases of valve migration to the ascending or descending aorta, which occurred within a few cardiac cycles following deployment. Although the performance of a balloon-expandable prosthesis in a heterotopic setting appears to be analogous with those of the self-expanding CRS2-4, two significant technical differences need to be underlined. Firstly, an Edwards SAPIEN dislocation can be managed with relative ease as long as coaxial wire position is maintained thereby preventing the prosthesis from rotating and turning over in the aorta obstructing normal aortic flow. The prosthesis can usually be directed into a secure and safe position using a partially inflated valvuloplasty balloon4. This situation is not encountered in CRS dislocation. Secondly, intentional valve dislocation can be achieved only with the CRS device. It follows that a severe paraprosthetic leak or coronary ostial impairment due to implant failure in an Edwards SAPIEN implant can be managed solely by implanting a second valve inside the first8,9 or with bailout left main stenting15,16, respectively.
Conclusions
Device dislocation represents a serious complication of TAVI, often necessitating the implantation of a second prosthesis. This strategy appears to be a feasible and effective interventional option. There seem to be no long-term vascular or neurological adverse events associated with device dislocation or intentional relocation in experienced hands.
Conflict of interest statement
G.P. Ussia is a proctor for Medtronic. All other authors have no conflict of interest to declare.

