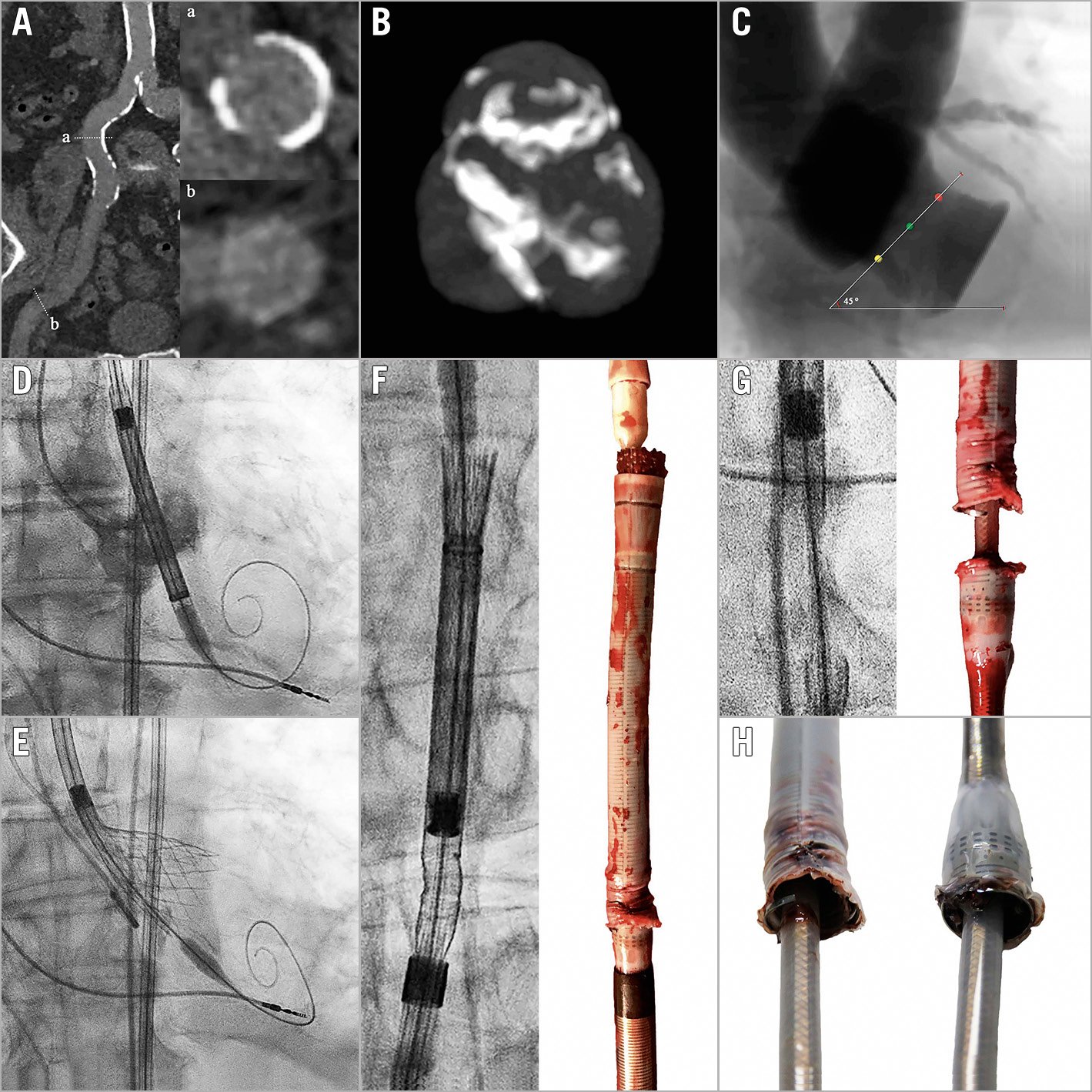
Figure 1. Delivery catheter system fracture during TAVI. A) Moderately calcified and tortuous iliofemoral arteries. B) & C) Moderately calcified and angulated trileaflet aortic valve. D) & E) Positioning, deployment and recapture of a 34 mm Evolut R valve. F) – H) Fractured delivery catheter system. Reproduced with permission from Medtronic.
An 87-year-old male with severe symptomatic aortic stenosis was accepted for transcatheter aortic valve implantation (TAVI) via transfemoral approach (Figure 1). Multislice computed tomo-graphy demonstrated moderately calcified and tortuous iliofemoral arteries (minimal lumen diameter 10.4 mm) (Figure 1A), and a moderately calcified and angulated trileaflet aortic valve (aortic annulus perimeter 85.0 mm; aortic root angulation 45º) (Figure 1B, Figure 1C), suitable for implantation of a 34 mm Evolut™ R valve (Medtronic, Minneapolis, MN, USA) as per standard practice at our centre. After vascular pre-closure with two Perclose devices (Abbott Vascular, Santa Clara, CA, USA) and valve predilatation with a 24 mm NuCLEUS™ balloon (NuMED, Cornwall, ON, Canada), a 16 Fr EnVeo™ PRO delivery catheter system (DCS) (Medtronic) easily traversed the iliofemoral vessels and the aortic valve. Deployment of the transcatheter heart valve (THV) was challenging due to recurrent migration towards the left ventricle, despite guidewire manipulation and rapid ventricular pacing (Figure 1D, Figure 1E). During a third recapture manoeuvre, the THV would not completely re-enter the DCS capsule, and further rotation of the delivery handle (either direction) did not move the THV. The DCS was retrieved into the descending aorta where fluoroscopy revealed a fracture at the proximal end of the capsule (Figure 1F-Figure 1H). As the DCS was attached to its capsule, it was possible to remove the entire DCS en bloc via the transfemoral access by gentle retraction, without the requirement for additional manoeuvres. Surgical back-up was on stand-by in case of access-site complications. Haemostasis was achieved using the Perclose sutures. The patient suffered no additional complications and was successfully treated with a 29 mm SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA) one month later.
Technical analysis of the DCS by the manufacturer confirmed complete fracture of the capsule and delamination of the nitinol-reinforcing frame. The DCS was otherwise intact and functioning appropriately. As no definitive cause was confirmed, a multifactorial origin was suggested. Potential causes include excessive tension and torque on the DCS due to anatomical factors (vascular calcification, tortuosity and angulation) and repeated recapture or over-capture of the THV. As such, careful patient selection and device manipulation during deployment and retrieval are advised, and adherence to the manufacturer recommendations of a maximum of three recapture manoeuvres is advised.
Conflict of interest statement
D. Mylotte is a consultant for Medtronic, Boston Scientific, and MicroPort. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Delivery catheter system advance.
Moving image 2. Transcatheter heart valve deployment and recapture.
Moving image 3. Delivery catheter system removal.

