CASE SUMMARY
BACKGROUND: An 81-year-old woman with symptomatic severe aortic stenosis and significant comorbidities was selected for transcatheter aortic valve implantation (TAVI).
INVESTIGATION: Transoesophageal echocardiography, multidetector row computed tomography, angiography.
DIAGNOSIS: Cardiac tamponade due to acute aortic annulus rupture during TAVI using an Edwards SAPIEN XT® prosthesis.
MANAGEMENT: Pericardiocentesis and valve-in-valve bailout procedure.
KEYWORDS: annulus rupture, aortic stenosis, complication, TAVI, valve-in-valve.
PRESENTATION OF THE CASE
An 81-year-old female patient with symptomatic degenerative severe aortic stenosis (indexed aortic valve area 0.4 cm2/m2, mean pressure gradient 43 mmHg) was selected for TAVI by the heart team due to frailty and presence of significant comorbidities, including severe pulmonary hypertension, rendering the patient at high surgical risk. (Logistic EuroSCORE I 24.5%, STS score 7.4%).
Preprocedural work-up included transoesophageal echocardiography (TOE) and multidetector row computed tomography (MDCT) of the aortic root, performed by the referring centre. The aortic valve was tricuspid and mildly calcified. The end-systolic aortic annulus diameter was 24.2 mm on TOE long-axis view whereas MDCT revealed an ellipsoid annulus with minimal and maximal diameter of 21.6 and 26.0 mm, respectively (Figure 1A-Figure 1C). In line with current recommendations and manufacturer guidelines, a 26 mm Edwards SAPIEN XT® prosthesis (Edwards Lifesciences, Irvine, CA, USA) was selected.
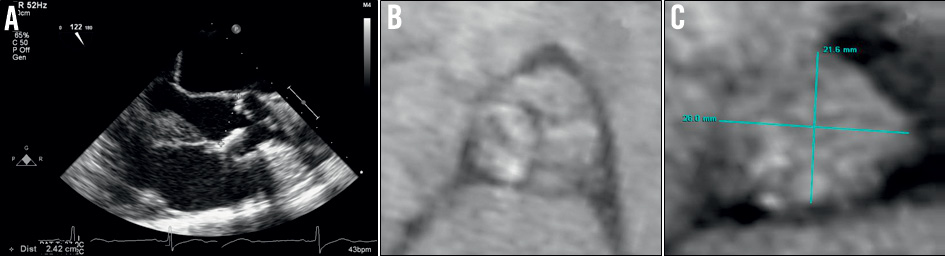
Figure 1. Preprocedural work-up. A) Aortic annulus of 24.2 mm at end-systole on long-axis view during transoesophageal echocardiography. B) Mildly calcified aortic valve on multidetector row computed tomography (MDCT). C) Ellipsoid aortic annulus, measuring 21.6×26 mm on MDCT.
Under general anaesthesia the patient first underwent a single percutaneous aortic valve dilatation, using a 23 mm balloon. Subsequently, using a right transfemoral route, the balloon-expandable Edwards SAPIEN XT® prosthesis was successfully implanted under rapid pacing. A few moments later, however, haemodynamic instability occurred and pericardial effusion was identified on left ventricular short-axis view during TOE (Figure 2A). In addition, urgent left ventricular angiography revealed extravasation of contrast agent just below the rim of the prosthetic frame (Figure 2B, Moving image 1). In conclusion, we were confronted with cardiac tamponade due to aortic annular rupture as a complication of TAVI.
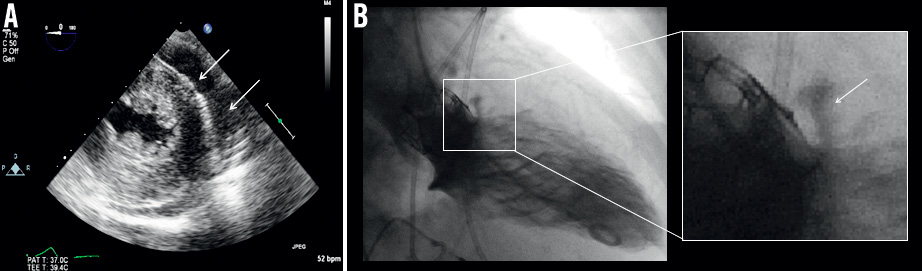
Figure 2. Cardiac tamponade due to acute aortic annular rupture. A) Pericardial effusion is noted on short-axis view of left ventricle during transoesophageal echocardiography (arrows). B) Left ventricular angiography shows contrast agent extravasation at the inferior rim of the prosthesis, indicating aortic annular rupture (arrow on right detail).
How would I treat?
THE INVITED EXPERTS’ OPINION
In order to guide management we need to understand the patient characteristics in some detail. From the presentation we will assume that this lady was at high but not extreme risk and would therefore potentially have been a candidate for conventional surgery.
The team had the advantage of TOE, which will have facilitated early diagnosis of tamponade. The initial management plan would be to resuscitate the patient and insert a percutaneous pericardial drain.
Subsequent management would depend on how stable the patient was from a haemodynamic point of view and the rate of blood loss through the drain. Given the patient factors, we would have a very low threshold for performing a median sternotomy to minimise the duration of haemodynamic decompensation and the degree of blood loss. Unless there were massive annular disruption, one would probably be able to achieve local control with packing to create time to facilitate discussion/planning regarding further more definitive management.
If there were massive annular disruption, one would be very unlikely to achieve haemodynamic stability. Under these circumstances we would perform an urgent sternotomy, rapidly establish cardiopulmonary bypass and convert to an open operation with excision of the valve, annular repair and aortic valve replacement (or aortic root replacement if required).
If this were a less severe posterior leak that was clearly identifiable and easily controlled by packing, the options would be:
1. Place some pledgeted sutures, reverse anticoagulation and transfuse platelets, pack locally with adjunctive haemostatic support (FlLOSEAL and/or TISSEEL; Baxter, Newbury, UK) and control the systolic BP to <110 mmHg. (Minor degrees of annular disruption –especially posteriorly– are uncommon but do occur after conventional surgery. These often resolve with local packing and reversal of anticoagulation).
OR
2. Given the position of the disruption on the angiogram (at lower end of stent) a further SAPIEN valve could be placed in a more ventricular position in an attempt to seal the leak.
We would favour the first of the above options as one could always revert to option two if it did not work. Also, the second option would have the attendant risk of converting the situation into one of massive annular disruption and is not something we would recommend without being ready to institute CPB immediately.
Any of the above measures should be followed by meticulous control of the blood pressure for the first week or so post procedure. Consideration should be given to avoiding any anticoagulant or antiplatelet therapy for a number of days.
Conflict of interest statement
N. Moat is a consultant and proctor to Medtronic. The other authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
Aortic root rupture (ARR) with cardiac tamponade and haemodynamic collapse during transcatheter aortic valve implantation (TAVI) is a devastating complication for both patients and physicians. Though relatively infrequent (0.5-1.0%), mortality is approximately 50%1,2. Rupture occurs most commonly at the inferior portion of the aortic annulus, an area described as a “vulnerable zone” due to the absence of external supporting structures and the proximity of the pericardial cavity3. Depending on its location, rupture may result in a ventricular septal defect, left ventricle to left atrial or right atrial shunt, or communication with the extracardiac space. The exact mechanical mechanism of ARR is unknown, but may be due to balloon overexpansion of the aortic root either during predilatation or valve deployment, displacement of calcified nodules, or differential tissue compliance.
Of course, prevention is better than cure. Thus, appreciation of possible factors associated with ARR is key. These include: excessive prosthesis oversizing; heavy valve calcification; balloon post-dilatation; and ellipsoid annuli. In the current case, the valve is not overtly calcified, but is ellipsoid: thus the choice of a 26 mm Edwards SAPIEN valve yields 16.9% and 0% oversizing for the short and long diameters. Certainly, the valve appears appropriate for the annulus post deployment.
Prompt identification of ARR is a crucial, yet frequently missed diagnosis2. Rupture should always be considered in the differential diagnosis of persistent hypotension during TAVI and mandates rapid echocardiographic or angiographic evaluation of the left ventricular outflow tract (LVOT) and aortic root. Immediate haemodynamic support should be initiated with delivery of vasopressors, colloid and blood products via large-bore central venous catheters. Anticoagulation should be reversed, and both platelet transfusion and emergent pericardiocentesis considered. Definitive treatment can potentially be achieved in three ways:
1. If the rupture is distal to the valve frame and likely to be due to balloon overexpansion of the LVOT, a second balloon dilatation could seal the rupture. Specially designed LVOT perfusion “doughnut” balloons which could potentially seal the rupture are in development, though are untested.
2. Implantation of a second transcatheter valve (valve-in-valve) at a lower position within the root may allow the second valve skirt to seal the rupture.
3. Finally, the above manoeuvres should be performed while the patient is being prepared for emergent cardiopulmonary bypass and corrective surgery.
In the current case, the ARR may be due to balloon overexpansion or incomplete valve deployment due to the presence of a calcified nodule. Thus, in the opinion of the authors, an attempt to seal the ARR by implantation of a second transcatheter valve is appropriate and may be life-saving.
Conflict of interest statement
N. Piazza is a consultant for Medtronic. The other authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF CASE
The cardiac tamponade was treated with emergent percutaneous pericardiocentesis and intravenous inotropes were administered. Subsequently, given the overall infaust outcome of acute surgery in this setting and given the clinical characteristics of the patient, including associated comorbidities, systemic frailty and acute haemodynamic compromise, immediate conversion to surgery was contraindicated after heart team discussion. As bailout, a percutaneous valve-in-valve procedure was performed as an attempt to seal the aortic annular rupture. A second Edwards SAPIEN XT® 26 mm prosthesis, using the same transfemoral access, was carefully positioned more distal to the first prosthesis and deployed at the level of the aortic annulus rupture (Figure 3A, Moving image 2). Subsequent left ventricular angiography did not show residual contrast agent extravasation, indicating adequate sealing of the aortic annular rupture (Figure 3B, Moving image 3). This bailout procedure finally resulted in the presence of two sequentially functioning prosthetic aortic valves (Figure 3C, Figure 3D, Moving image 4). After cardiopulmonary resuscitation for 20 minutes following the second prosthesis placement, the patient was stabilised under further inotropic support and transferred to the intensive care unit. Six hours later, haemodynamic deterioration re-occurred with increase of pericardial effusion and the patient deceased due to cardiogenic shock.
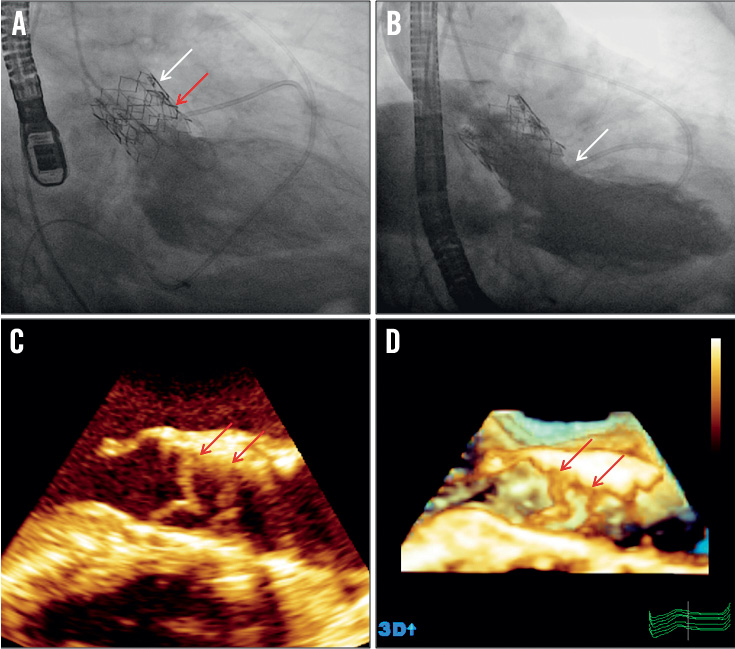
Figure 3. Valve-in-valve bailout procedure to treat aortic annular rupture. A) Distal to the first prosthesis (white arrow) a second prosthesis is positioned and deployed (red arrow). B) Absence of contrast agent extravasation (arrow) on left ventricular angiography indicates sealed aortic annular rupture. C) 2-dimensional echocardiography and D) 3-dimensional echocardiography long-axis view shows presence of two sequentially functioning prosthetic aortic valves (arrows).
Discussion
This case highlights several important clinical issues regarding prevention and management of acute complications during TAVI.
PROSTHESIS SIZING AMBIGUITIES
Accurate prosthesis sizing is a critical determinant of acute and long-term TAVI outcome. The incidence of device landing zone rupture (including annular rupture) during TAVI is almost 1%1,2. In a recent series including 618 patients treated with TAVI, this complication was described in six patients3. The oversizing of the aortic prosthesis relative to the measured annular size was identified as a key determinant for the occurrence of this complication, especially when precipitating factors such as prominent calcifications, sequential balloon expansions or small aortic root coincided. In the present case report, an aortic annular diameter of 24.2 mm on TOE and a maximal diameter of 26.0 mm on MDCT led us to choose a 26 mm prosthesis, in line with current recommendations4. However, MDCT revealed an ellipsoid annulus with minimal diameter of 21.6 mm. In addition, post-factum measurement of the aortic annular perimeter (7.17 cm) and area (4.02 cm²) on MDCT were more in line with the nominal sizes of a 23 mm aortic valve prosthesis (7.2 cm and 4.15 cm², respectively) compared to a 26 mm prosthesis (8.2 cm and 5.31 cm², respectively). Therefore, use of a 23 mm aortic valve prosthesis might have been more appropriate and might avoid excessive oversizing and subsequent annular rupture. Current recommendations for prosthesis sizing are based on two-dimensional measurements of the aortic annulus diameter, but these have been shown to underestimate true annular size when compared to the use of three-dimensional imaging techniques which show a more ellipsoid rather than a circular aortic annulus in the vast majority of patients and which provide additional aortic annular dimensions, such as perimeter and area, which can help to select the most appropriate prosthesis size5. Several series have demonstrated that three-dimensional imaging techniques provide more accurate anatomical information of the aortic annulus compared to two-dimensional echocardiography-based measurements6. So far, the gold-standard method to size the aortic valve annulus has not been established and the dimensions used to select the prosthesis still rely on aortic annulus diameter despite perimeter or area perhaps being more helpful. Although recently the relative prosthesis oversizing in relation to MDCT-based aortic annular measurements has been advocated to reduce postprocedural paravalvular aortic regurgitation in TAVI, maximal cut-off values associated with aortic annulus rupture should be assessed7,8. Clearly, there is a current unmet clinical need for a prospective and randomised study to define the gold-standard aortic annular measurement (diameter, perimeter, area) and to determine the ideal imaging modality for accurate prosthesis sizing.
DIAGNOSING ACUTE TAVI COMPLICATIONS
Use of TOE during TAVI permits immediate diagnosis of procedural complications such as aortic annulus rupture, ventricular perforation or aortic rupture/dissection that should be suspected if cardiac tamponade occurs2,9. In addition, angiography of the aortic root and left ventricular outflow tract should be performed2,9. Contrast extravasation at the inferior rim of the prosthesis was diagnostic for aortic annulus rupture in this case.
USE OF PERCUTANEOUS BAILOUT PROCEDURES
Conversion to acute surgery for aortic annular rupture during TAVI is associated with poor survival2,3,9. Hence, percutaneous bailout procedures to treat this life-threatening complication need consideration. To the best of our knowledge, this is the first report indicating that a valve-in-valve procedure may seal aortic annular rupture and provides, at least temporary, life-saving therapy. Therefore, it may represent a bridge to (less acute) surgery, potentially improving outcome in this very high-risk population.
Funding
P. Debonnaire is supported by a Sandra Medical Research Grant (Boston Scientific). The Department of Cardiology of Leiden University Medical Center received grants from GE Healthcare, Lantheus Medical Imaging, St. Jude Medical, Medtronic, Boston Scientific, Biotronik, and Edwards Lifesciences.
Conflict of interest statement
V. Delgado received consulting fees from St. Jude Medical and Medtronic. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Left ventricular angiography reveals contrast agent extravasation below the rim of the aortic valve prosthesis due to acute aortic annulus rupture.
Moving image 2. Deployment of valve-in-valve as bailout treatment.
Moving image 3. Absence of contrast extravasation after valve-in-valve bailout procedure, indicating sealing of aortic annular rupture.
Moving image 4. Three-dimensional echocardiography showing presence of two sequentially functioning prosthetic aortic valves.

