
Abstract
Aims: Long-term outcomes are available for first-generation transcatheter heart valves but data on second-generation devices are scarce. We aimed to provide an oversight of all patients implanted with a second-generation valve in our centre.
Methods and results: From April 2012 to July 2016, 219 patients were enrolled in this prospective single-centre experience; they received either the transapical ACURATE TA (n=99) or the transfemoral ACURATE neo (n=120) prosthesis. Data were collected during the hospital stay and telephone follow-ups were conducted at 30 days post procedure and annually thereafter. Patients were 80.9±4.4 years old with a mean logistic EuroSCORE I of 19.3±13.9%. Transapical patients had significantly more comorbidities at baseline. Post intervention, mean gradient was reduced to 10.6±9.2 mmHg, and 1.9% had moderate paravalvular regurgitation. Mean follow-up time, based on the last patient contact, was 217±188 days for the transfemoral and 525±413 days for the transapical group. Thirty-day mortality was 2.5% and 4.0%, and one-year Kaplan-Meier survival was 94.8% (95% CI: 87.5-97.9) and 81.9% (95% CI: 72.0-88.5), respectively. At two years, survival was 64.9% (95% CI: 52.6-74.7) for transapical patients.
Conclusions: This early single-centre experience showed very good safety and performance outcomes in patients treated with the ACURATE prostheses.
Abbreviations
CT: computed tomography
NYHA: New York Heart Association
PVL: paravalvular leakage
sAVR: surgical aortic valve replacement
TAVI: transcatheter aortic valve implantation
Introduction
Transcatheter aortic valve implantation (TAVI) has been developed as an alternative therapy to treat patients with severe symptomatic aortic stenosis who are at high risk for surgical aortic valve replacement (sAVR). The value of TAVI has been confirmed in randomised controlled trials, showing superior one-year survival rates of TAVI compared to sAVR in high-risk patients1,2. Long-term data are available for first-generation devices3,4.
Second-generation devices have shown promising early outcomes, but long-term data are scarce. The ACURATE™ transcatheter heart valve system (Symetis SA, Eclubens, Switzerland) is such a next-generation device with reported outcomes up to one year5-8. It gained a CE mark in 2011 for transapical (ACURATE TA™) and in 2014 for transfemoral access (ACURATE neo™).
This report provides an overview of all consecutive patients with aortic stenosis in whom an implant with an ACURATE valve was attempted at our centre. To the best of our knowledge, this is the largest published single-centre series of this prosthesis to date, and the only published report with data beyond one year.
Methods
This prospective single-centre data collection included all consecutive patients in whom an ACURATE implant was attempted to treat aortic stenosis. Patients treated for predominant aortic regurgitation were excluded. Data were collected during the hospital stay. Telephone follow-ups were conducted at 30 days and at yearly intervals.
The assignment to TAVI, valve and access selection was performed by a Heart Team. We used a transfemoral first strategy but, in case of suboptimal vascular access, we liberally selected the transapical route. At the beginning of our experience, valve sizing was performed integrating information from computed tomography (CT) and transoesophageal echocardiography. Since 2014 we have exclusively used CT data analysed with the 3mensio software (Pie Medical Imaging BV, Maastricht, the Netherlands) to determine annulus perimeter and area for valve sizing. We recommended dual antiplatelet therapy with acetylsalicylic acid and clopidogrel for three months; in case of systemic anticoagulation only clopidogrel was administered.
The ACURATE systems have been described previously5,6,8,9. In brief, both bioprostheses consist of a self-expanding nitinol stent with an anatomic conformability to the native annular shape. Stabilisation arches are designed to pivot against the ascending aorta for self-alignment of the prosthesis. The upper crown allows supra-annular anchoring, stable positioning and tactile feedback. A skirt acts as a seal to prevent paravalvular leakage (PVL). The delivery system is designed for single operator deployment. The ACURATE TA valve is made of three non-coronary native porcine leaflets, while the ACURATE neo valve is made of porcine pericardial tissue with BioFix™ anticalcification treatment. The ACURATE neo is a supra-annular valve and its delivery system is compatible with 15 Fr balloon-expandable and 18 Fr rigid sheaths. We used either an 18 Fr Cook sheath (Cook Medical, Bloomington, IN, USA) or the inner 14 Fr lining of a Transglide 18 Fr sheath (TransAortic Medical, Inc., Morgan Hill, CA, USA). Predilatation was generally performed and rapid pacing applied. Valve release was usually performed without rapid pacing. Post-dilatation was considered in case of unfavourable results with significant (≥2+) PVL.
Degree and distribution of calcification was assessed with the 3mensio software using a pre-set window of >450 Hounsfield units. Analysis was performed visually by an experienced interventional cardiologist with the use of a qualitative grading system from none, mild, moderate to severe. Clinical outcomes were reported using the VARC-2 criteria unless otherwise specified10.
Statistical analysis
The statistical analysis is based on the intention-to-treat principle and includes all patients in whom an implant was attempted. The follow-up time was calculated from the procedure date to the last available subject information. Categorical variables are expressed as numbers and percentages of the total. Continuous variables are expressed as mean±SD. Confidence intervals were calculated as appropriate. Groups were compared using the Pearson’s χ² test, t-test, and Kaplan-Meier survival analysis using the log-rank test. All statistical analyses were performed using SPSS, Version 23 (IBM Corp., Armonk, NY, USA).
Results
We prospectively collected data on all ACURATE implants, starting from our first implant in April 2012, until July 2016; the first transfemoral ACURATE neo implantation was performed in November 2014. During that time, approximately one third of our transapical patients and nearly half of our transfemoral patients received the ACURATE prostheses. Prior to the introduction of the Symetis valve, we had a three-year TAVI experience with 312 valves implanted (predominantly balloon-expandable prostheses).
Diabetes mellitus was present in 34.2% of patients (75/219), atrial fibrillation in 28.0% (61/218), 15.1% (33/219) had prior cerebrovascular accidents, and 39.2% (85/217) moderate or severely reduced ejection fraction. Transapical patients had a significantly higher logistic EuroSCORE I and higher New York Heart Association (NYHA) classification, and significantly more frequently had diabetes mellitus, atrial fibrillation, preoperative dialysis, reduced ejection fraction and prior cardiac surgeries. Cusp calcification was more severe in the transfemoral group (Table 1).
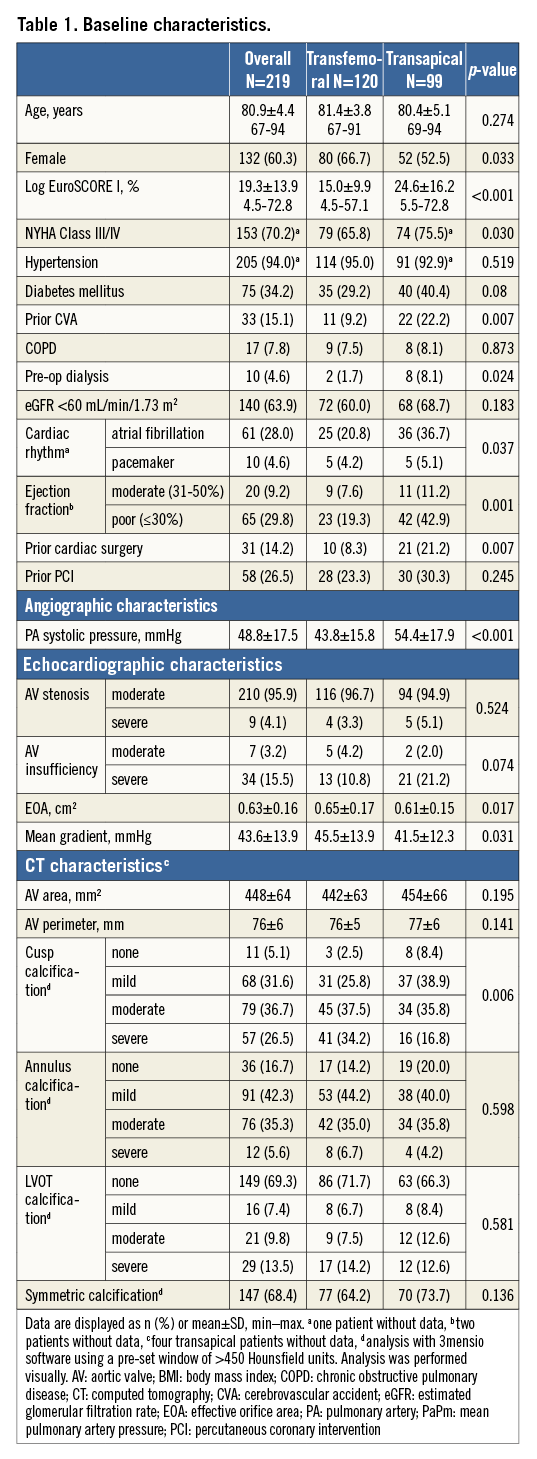
All but two transfemoral patients were treated under general anaesthesia. We started implanting the prosthesis under rapid pacing according to the instructions for use, but later we began to deploy all without rapid pacing. Post-dilatation was more frequent in the transfemoral group (53.3% versus 37.4%, p=0.018) (Table 2). Usage of the heart-lung machine was required in two transapical cases, one for life-threatening apical bleeding due to a tear of the purse-string suture with subsequent sternotomy, and one left ventricular decompensation in a 93-year-old patient with a logistic EuroSCORE I of 54.6%, NYHA Class IV, and prior pacemaker implantation. Both patients subsequently died. Valve-in-valve procedures for aortic regurgitation were necessary in four (1.8%) patients and were performed with a SAPIEN 3 prosthesis (Edwards Lifesciences, Irvine, CA, USA).
An echocardiographic assessment was conducted prior to discharge. No or mild PVL was observed in 98.1% (210/214), moderate in 1.9% (4/214), and severe in none (Figure 1). Mean gradient was 10.6±9.2 mmHg (95% CI: 9.3-10.6) and was lower in the transfemoral compared to the transapical group (8.3±4.0 mmHg compared to 12.3±6.3 mmHg, p<0.001).
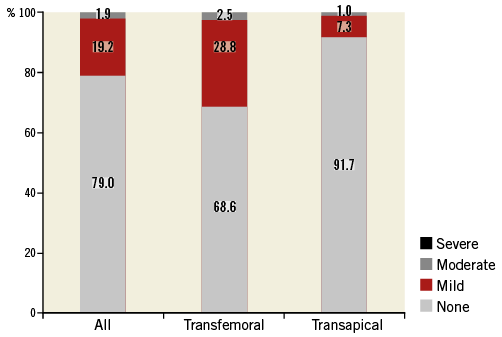
Figure 1. Post-procedural paravalvular leak assessment. The transfemoral group had significantly higher rates of paravalvular leakage compared to the transapical group.
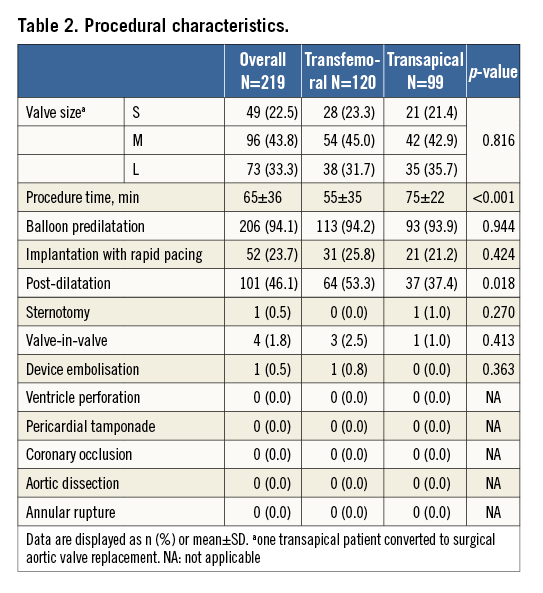
Clinical outcomes are provided in Table 3. Thirty-day mortality was 2.5% in the transfemoral and 4.0% in the transapical group (p=0.541). Six strokes occurred (2.7%): of these, one was non-disabling with a modified Rankin scale score of 1. Two strokes were evident immediately after the procedure; the other strokes occurred on day 2, 4, 5, and 9 post procedure. One additional stroke occurred on day 31. Of the six patients experiencing an early stroke, atrial fibrillation with a preoperative thrombus in the left atrial appendage was present in three patients, one had a history of prior stroke and one a valve-in-valve implantation. Permanent pacemakers were implanted in 27 patients (12.3%), of which 23 for AV block, three for bradyarrhythmias and one for sick sinus syndrome. Two ventricular septal defects occurred, one of which required intervention. Both patients had severe calcification of the left ventricular outflow tract and post-dilatation was required to treat PVL; the patients were alive at their last follow-up at one and three years, respectively. Mean follow-up time, based on the time of last patient contact, was 525±413 days for transapical and 217±188 days for transfemoral patients. During follow-up, we did not detect any evidence of early valve failure. With the caveat of only 46 patients at risk, one-year survival was 94.8% (95% CI: 87.5-97.9) in the transfemoral group. For transapical patients, survival was 81.9% (95% CI: 72.0-88.5) at one year and 64.9% (95% CI: 52.6-74.7) at two years (Figure 2).
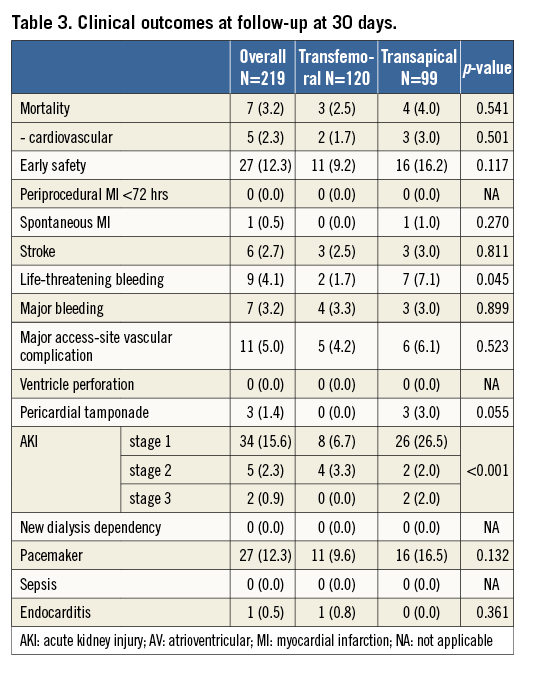
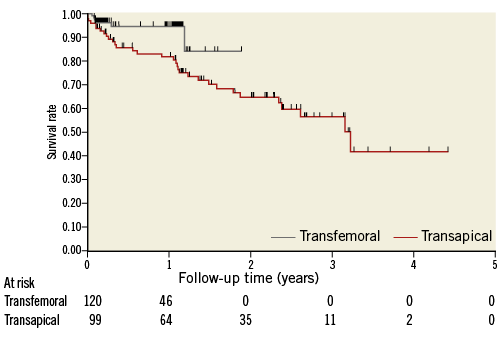
Figure 2. Kaplan-Meier survival by treatment group. All-cause mortality was significantly higher in the transapical group (log-rank=0.018).
Discussion
The main findings of our series are: (i) very good clinical and performance outcomes in patients implanted with a next-generation self-expanding transcatheter heart valve despite the inclusion of the first patients implanted with this device, reflecting a swift learning curve, (ii) good long-term survival, and (iii) transapical patients were more severely diseased than transfemoral patients, which impacted on survival.
Our patients were a typical TAVI population characterised by a high logistic EuroSCORE I and high rates of prior cerebrovascular accidents, prior cardiac surgery, atrial fibrillation, and poor ejection fraction, especially in the transapical group. The aortic valve parameters reflect the valve selection at our centre which particularly favours the ACURATE valve in unfavourable anatomic situations such as no, mild or asymmetric cusp and annular calcification, or heavy calcification of the left ventricular outflow tract. Usually, a certain amount of calcium is required to anchor the prosthesis and to avoid valve migration, but due to the specific design features of the ACURATE prosthesis, such as the diabolo shape facilitating self-alignment and self-centring8, calcification for anchoring is helpful but not indispensable. For heavy calcifications of the left ventricular outflow tract, we refrain from using balloon-expandable valves to avoid the risk of calcium protrusion, annular rupture, or ventricular septal defects.
We treated nearly all patients under general anaesthesia and have recently started to use conscious sedation. Similar to other series8, we started our experience with rapid pacing but, after we learned that the stabilisation arches reliably stabilise the valve, we now perform the implantation without rapid pacing. Only in cases of large excursions of the annular plane during the heart cycle do we use fast pacing at 100-120 bpm to reduce movement during final deployment. Despite the fact that our series starts from the first patients implanted, outcomes have been robust, which speaks for the frequently claimed “ease of use” of the device5,6,9. Further, as we used the ACURATE valves in difficult anatomies, this might have induced a bias towards negative results. Nevertheless, bail-out procedures were rare (1.8% valve-in-valve procedures and 0.5% device embolisation), and no aortic dissection or annular rupture was observed. Post-dilatation was more frequently performed with the transfemoral ACURATE neo valve which might be related to the higher radial strength of the transapical ACURATE TA valve, but may also be caused by a higher degree of cusp calcification in transfemoral patients at baseline.
Echocardiographic outcomes are in line with those previously reported. The mean gradient was slightly lower in the transfemoral ACURATE neo group which might be caused by the true supra-annular design of this valve. Conversely, the ACURATE TA group had slightly less PVL ≥2+ (1.0% compared to 2.5%), which might – as with the post-dilatation – be caused either by the higher radial force of the prosthesis or by the lower degree of cusp calcification compared to transfemoral patients. Nevertheless, the rate is still very low, comparable to the SAPIEN 3 and CoreValve® Evolut™ R (Medtronic, Minneapolis, MN, USA) (2.6-3.5%)11-13 and superior to the CoreValve prosthesis (7.3% in the CoreValve US and 12.9% in the Advance study)1,14. Certainly, the lack of core laboratory data is a limitation, but our results are in line with those previously reported for the ACURATE valves ranging from 0% to 3.4%5,7,8,15,16. Furthermore, our findings are confirmed in a case-matched study, which reported superior performance of the ACURATE neo compared to the CoreValve prosthesis7, and a propensity score-matched analysis, which reported comparable results of the ACURATE TA and the SAPIEN XT (Edwards Lifesciences)16.
While our PVL rate is comparable to that of other trials using the same valve, we observed a slightly higher pacemaker rate than expected in the transapical group (16.5% compared to approximately 10% in the “TA90” cohort and SAVI registries6,8), which might be due to a liberal use of pacemakers at our centre. Transfemoral patients had numerically fewer pacemakers (9.2%). Again, this might be caused by the higher radial strength of ACURATE TA or differences in baseline characteristics. Notably, the pacemaker rate in the transfemoral group is comparable to that of balloon-expandable valves (12.5-14.8% for SAPIEN 3)11,12.
The 30-day stroke rate of 2.7% is low, confirming the safety of the device. We barely (approx. <2%) use cerebrovascular protection devices as, in more than 1,000 patients implanted with TAVI devices, we have only experienced three intraprocedural strokes. Considering that, in this series, no patient had an intraprocedural stroke, and only two out of six patients an acute stroke within 24 hours of procedure, one could assume that the high rate of comorbidities (atrial fibrillation, left atrial appendage thrombi, kidney disease, prior stroke) could have negatively impacted on the stroke rate. In a recent systematic review, kidney disease and new onset of atrial fibrillation were predictive for cerebrovascular events, as well as early device experience17. Also, left atrial appendage thrombus embolisation is a potential mechanism of stroke, which might merit particular attention to the anticoagulation regime and eventually embolic protection devices18. Notably, intracardiac mass is an exclusion criterion in several clinical studies (e.g., ClinicalTrials.gov #NCT01808287, NCT01314313, NCT01240902). The effect of post-dilatation on cerebrovascular events is the subject of controversial discussion. While a recent review of 64 studies with 72,318 patients showed only a non-significant trend towards higher cerebrovascular events in patients treated with post-dilatation, others showed an increased stroke risk, but only for early (≤7 days), and particularly acute (≤24 hours) strokes17,19,20.
Considering the early series, the rate of major vascular complications was low. This might have been caused by our conservative approach, transferring patients to transapical therapy when in doubt. Further, our technique to use the inner 14 Fr lining of a Transglide 18 Fr sheath might have influenced outcomes. One ventricular septal defect requiring intervention was detected 10 weeks post procedure in a patient with heavy calcification of the ascending aorta after mediastinal radiation. The defect was successfully treated with an AMPLATZER™ PFO Occluder (St. Jude Medical, St. Paul, MN, USA) under surgical visualisation21.
The 30-day mortality rate was very low and similar amongst the groups, yet there was a difference in one-year survival, which is not surprising given the differences in baseline characteristics. Even though we included all patients starting from our first ACURATE implant, our outcomes are comparable with those of the SAPIEN 3 and CoreValve prosthesis. In particular, the 30-day mortality rate for transfemoral patients was 2.5% compared to 2.1-4.5% (0% in 60 patients treated with the CoreValve Evolut R prosthesis13), and 4.0% compared to 11.1% for transapical/transaortic patients; one-year survival was 94.8% compared to 85.8-88.9% and 81.9% compared to 74.7%, respectively1,11,12,14. Survival data beyond one year are only available for a limited number of transapical patients. In particular, survival was 64.9% at two years, which is similar to that of transapical patients in a recently reported multicentre experience (74.9%), to high-risk PARTNER cohort A patients including transfemoral and transapical patients (66.1%), and to transfemoral CoreValve patients (68-71%)4,22-24.
Limitations
Limitations are those inherent to a single-centre registry. We did not use a core laboratory and endpoints were not adjudicated. The comparison to other valves is hampered as we used the ACURATE prosthesis mostly in difficult anatomies. Since enrolment spanned over four years, treatment and patient selection patterns changed over time. Major limitations are the limited number of patients at long-term follow-up and the absence of echocardiographic follow-up.
Conclusions
In a four-year single-centre experience starting from the first patient implanted with an ACURATE transcatheter heart valve system, clinical and performance outcomes were very good and comparable to contemporary devices. We use the ACURATE prosthesis by default in none/mild calcification as well as severe calcification of the left ventricular outflow tract. For a “normal” anatomical situation, we do not have sufficient evidence to favour a specific prosthesis. Due to the lack of systematic long-term echocardiographic follow-up, we cannot determine the longevity of the prosthesis, but it is encouraging that we did not observe any signs of valve failure at follow-up.
| Impact on daily practice This is the first published ACURATE experience with data beyond one year. It confirms that the device is easy to use and can be successfully implanted with very good procedural outcomes even in difficult anatomies such as little or severe calcification. Long-term survival was encouraging considering the high-risk patient population without any sign of early valve failure. |
Acknowledgements
We wish to thank Beatrix Doerr for her editorial assistance, reimbursed by Symetis SA.
Conflict of interest statement
The authors have no conflicts of interest to declare.

