
Abstract
The ACURATE neo aortic valve system is a self-expanding transcatheter device that was granted CE mark in 2014 and has since been widely adopted in the treatment of patients with severe aortic stenosis. The ACURATE neo can be used in a wide clinical spectrum, but there are some specific indications and anatomies where this device is particularly suitable. Recently, it was shown that, with appropriate patient screening, size selection, and optimised positioning, results can be improved substantially. This review provides an overview of existing data and compiles a standardised manual of best practice for the implantation of this device based on both evidence and individual experience.
Introduction
The ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) is a self-expanding transcatheter heart valve with a supra-annular design and porcine pericardial leaflets that has been commercially available in Europe since 2014. It is characterised by a top-down deployment, which allows precise positioning and minimises flow obstruction during deployment1. Three stabilisation arches provide a better coaxial alignment, and the upper crown supports anchoring (Figure 1). The transfemoral delivery system incorporates two knobs in the handle that can be turned to deploy the device in two steps. The smallest diameter of the delivery system is 15 Fr, increasing to 18 Fr at the site of the valve attachment. The ACURATE neo valve can be implanted via either transvascular or transapical access routes and has a dedicated transapical delivery system. Since CE-mark approval in 2014, it has been widely adopted in Europe, Canada, South America, and the Asia-Pacific region. A recent study illustrated that results are considerably subject to appropriate patient screening, size selection, and optimised positioning2.
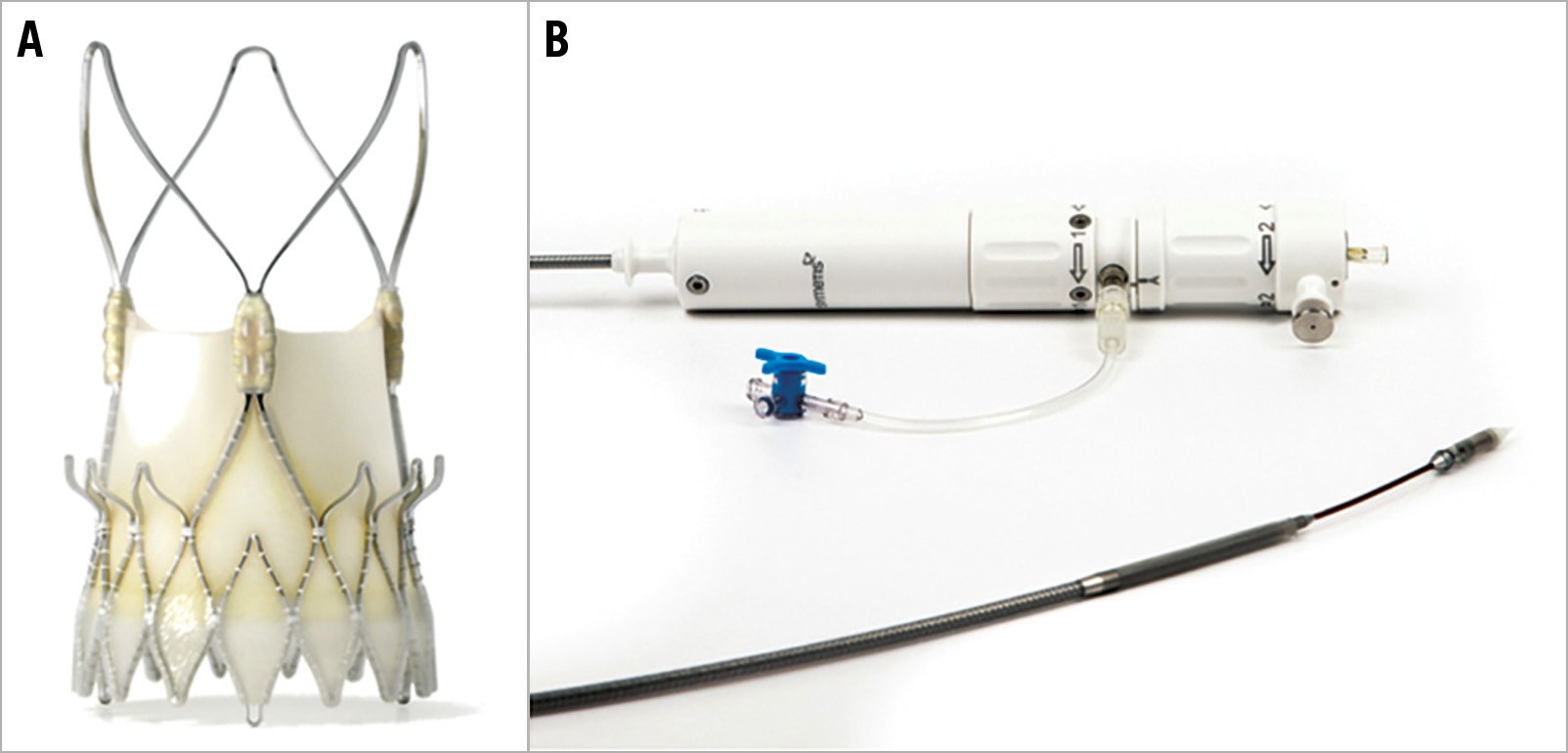
Figure 1. ACURATE neo transcatheter heart valve system. The ACURATE neo prosthesis (A) and delivery system (B).
The purpose of this manuscript is to review the growing amount of clinical data on the ACURATE neo and to present a sophisticated approach for sizing and patient selection by highlighting suitable anatomies and indications. Moreover, we aim to provide a best practice manual going through each procedural step of transfemoral implantation based on insights from experienced operators.
Clinical data
CE-MARK STUDY
This prospective series included the first 89 patients who were implanted with the ACURATE neo prosthesis (age 83.7±4.4 years; logistic EuroSCORE 26.5±7.7%)3. Procedural success was 94.4%. At 30 days, all-cause mortality was 3.4%, the rate of moderate paravalvular leakage (PVL) was 4.5%, major stroke occurred in 2.2%, and the frequency of permanent pacemaker implantation (PPI) was 10.3%.
SAVI TF REGISTRY
The purpose of this prospective, international registry was to demonstrate the efficacy and safety of the ACURATE neo in a real-world setting4. A total of 1,000 patients from 25 centres (age 81.1±5.2 years; STS score 6.0±5.6%) were included. Procedural success was obtained in 98.7%, mean gradient was 8.4±4.0 mmHg, and more-than-mild PVL 4.1%. At 30 days, all-cause mortality was 1.4%, PPI occurred in 8.3%, and there was no case of coronary obstruction requiring intervention.
MORENA
Data from three high-volume centres in Germany were merged for a comparison of the ACURATE neo and the balloon-expandable SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA)5. From a total of 1,121 patients, a matched cohort of patients (SAPIEN 3, n=622; ACURATE neo, n=311) was identified. Rates of in-hospital complications were similar between the groups, including major stroke, major vascular complications, and life-threatening bleeding. Thirty-day mortality (2.3% vs 1.9%; p=0.74) and overall device failure were similar (10.9% vs 9.6%; odds ratio: 1.09; p=0.71) between the groups, with increased rates of more-than-mild PVL (4.8% vs 1.8%; p=0.01), but less elevated gradients (3.2% vs 6.9%; p=0.02) and less frequent PPI (9.9% vs 15.5%; p=0.02) in the ACURATE neo group.
SCOPE I
In this randomised trial, the ACURATE neo was compared with the balloon-expandable SAPIEN 3 system for transfemoral TAVI in patients with severe aortic stenosis6. A total of 739 patients from 20 European centres (age 82.8±4.1 years; STS score 3.5%) were enrolled. At 30 days, the primary composite endpoint (all-cause death, any stroke, life-threatening or disabling bleeding, major vascular complications, coronary artery obstruction requiring intervention, acute kidney injury [stage 2 or 3], rehospitalisation for valve-related symptoms or congestive heart failure, valve-related dysfunction requiring repeat procedure, moderate or severe prosthetic valve regurgitation, or prosthetic valve stenosis) occurred in 24% in the ACURATE neo group and in 16% in the SAPIEN 3 group; thus, non-inferiority of the ACURATE neo was not met (absolute risk difference 7.1% [upper 95% confidence limit 12.0%], p=0.42), and the secondary analysis suggested superiority of the SAPIEN 3 THV over the ACURATE neo device (95% CI for risk difference −1.3 to −12.9, p=0.0156). While all-cause mortality and stroke rates were similar, more-than-mild PVL was more frequent in the ACURATE neo group (9% vs 3%).
NEOPRO REGISTRY
In this multicentre observational registry, 1,551 patients (mean age 82 years, STS score 5.1%) who underwent transfemoral TAVI with either the ACURATE neo (n=1,263) or the Evolut™ PRO (n=288) valve (Medtronic, Minneapolis, MN, USA) were included. After propensity score matching, device success (86.9% vs 89.0%, p=0.48), more-than-mild PVL (10.9% vs 8.5%, p=0.37), 30-day mortality (3.2% vs 1.2%, p=0.13), 30-day stroke (2.4% vs 2.8%, p=0.79), 30-day VARC-2 early safety endpoint (10.6% vs 10.4%, p=0.96), and new PPI (12.8% vs 11.9%, p=0.55) were similar between the groups7.
COMPARISON OF NEW-GENERATION DEVICES
A total of 346 patients (age 81.4±5.2 years; STS PROM 4.0±2.5%) from a single centre treated with a new-generation THV (SAPIEN 3 n=134; Evolut™ R [Medtronic] n=111, ACURATE neo n=101) were compared against each other. At 30 days, all-cause mortality was similar between groups, whereas rates of PVL and PPI and mean gradients differed significantly (SAPIEN 3 vs Evolut R vs ACURATE neo: more-than-trace PVL 18.8% vs 47.9% vs 45.8%, p<0.05; PPI 8.3% vs 16.7% vs 2.1%, p<0.05; pmean 9.7±7.5 mmHg vs 6.1±2.4 mmHg vs 8.4±3.5 mmHg, p<0.01). At one year, MACCE rates were similar among all groups8.
SMALL AORTIC ANNULI
In this multicentre study, a total of 92 matched pairs of patients with an aortic annulus area below 400 mm2 undergoing TAVI with either the supra-annular ACURATE neo or the intra-annular SAPIEN 3 prosthesis were studied9. The ACURATE neo provided a larger indexed effective orifice area (EOA) (0.96 cm²/m² vs 0.80 cm²/m²; p<0.001) and lower rates of severe patient-prosthesis mismatch (PPM) (3% vs 22%; p<0.001) as well as lower mean transvalvular gradients (9.3 mmHg vs 14.5 mmHg; p<0.001). These haemodynamic findings were sustained at one-year follow-up. Mortality at 30 days and one year, and in-hospital rates of stroke, PPI rate, as well as more-than-mild PVL were similar for the two THV systems.
PERMANENT PACEMAKER IMPLANTATION
In a small study that included 175 patients (83±6 years, STS score 4.1±2.4%) from three centres, the PPI rate using the ACURATE neo was as low as 2.5% in pacemaker-naïve patients10. The authors concluded that a less aggressive predilatation to minimise mechanical trauma to the conduction system, periprocedural avoidance of negative dromotropic drugs, and conservative indication for a new PPI may be strategies that help to achieve a low PPI rate.
SELECT RBBB
This recent multicentre study included 296 patients without previous pacemaker and pre-existing right bundle branch block from seven centres undergoing TAVI using either the ACURATE neo (n=98) or the SAPIEN 3 device (n=198). The 30-day PPI rate was lower when using the ACURATE neo (29.6% vs 43.9%; p=0.025; OR 0.54, 95% CI: 0.32-0.89; p=0.018). There was no difference in device failure (8.2% vs 6.6%; p=0.792)11.
PREDICTORS OF PVL
In a comprehensive analysis of anatomical and procedure-related factors of PVL in 500 patients (82.1 years; STS score 4.4%) undergoing transfemoral TAVI with the ACURATE neo in a single centre, more-than-mild PVL was more frequent with increasing device landing zone calcification (mild 0.8% vs moderate 5.0% vs severe 13.0%; p<0.001). The degree of peri-annular calcification, oversizing, presence of annular plaque protrusions, inappropriate positioning, and the sinotubular junction height were identified as independent predictors of more-than-mild PVL. When comparing the first 100 with the last 100 ACURATE neo cases performed in this centre, more-than-mild PVL decreased from 11% to 3% (p=0.03), an observation that was attributed to increased oversizing, selection of patients with less calcified aortic valve calcification, and improved positioning2.
BALLOON PREDILATATION
Given the comparably moderate radial force of the ACURATE neo, an effective balloon predilatation is mandatory. However, the feasibility and safety of direct implantation without predilatation were demonstrated in a single-centre series of selected patients with mild aortic valve calcification. From a total of 294 patients, 72 (24%) cases were performed without predilatation (82.7 years, STS score 4.6%). Device success (VARC-2) was achieved in 94.4%, post-dilation was necessary in 26.4%, and one (1.4%) patient had moderate PVL. A propensity-matched comparison of patients with versus without predilatation showed that there were no differences regarding device success, more-than-mild PVL, post-dilation, and post-procedural mean gradients, but procedure and fluoroscopy times were significantly decreased in the group without predilatation12.
PURE AORTIC REGURGITATION
The frame of the ACURATE neo has an X-shaped design with the upper crown being 5 mm larger than the nominal THV diameter at the waist. This may help to anchor the prosthesis and prevent embolisation into the left ventricle even in the absence of calcification. The current evidence is scarce, but a small series of 20 patients with pure aortic regurgitation showed favourable haemodynamic outcomes13. This study demonstrated that, due to the absence of calcification, more oversizing may be required compared to patients with aortic stenosis. The maximum mean diameter that was treated with the largest available size (ACURATE neo L, 27 mm) in the published series was 25 mm. Furthermore, to minimise the risk of ventricular embolisation, the initial positioning may be slightly higher than for the implantation in aortic stenosis, and rapid pacing may be used to enhance stability during deployment.
BICUSPID AORTIC VALVE
In a multicentre registry, among 712 patients who were treated with the ACURATE neo THV, a bicuspid aortic valve (BAV) was identified in 54 (7.5%) cases14. In comparison to patients with tricuspid anatomy (n=658; 92.4%), the presence of BAV was associated with more frequent post-dilatation (57.4% vs 38.7%, p=0.007), more-than-mild PVL (7.4% vs 3.2%, p<0.001), and major stroke (7.4% vs 1.8%, p=0.001). After propensity score matching, the rate of post-dilation remained higher in the BAV group, whereas more-than-mild PVL and major stroke were similar between groups.
In summary, a growing body of evidence demonstrates the feasibility and safety of using the ACURATE neo for approved indications, but also in off-label situations. The X-shaped design allows an optimal distribution of the relatively moderate radial force (Figure 2), which translates into a balanced profile of this valve with a low risk of annular rupture, coronary obstruction, and conduction disturbances whilst having an acceptable rate of more-than-mild PVL in most series. However, the unusually high frequency of more-than-mild PVL of 9% in the recent SCOPE I trial is inconsistent with previous data and requires further clarification, before a final recommendation regarding differential device selection can be made. These inconsistencies may be ascribed to the absent core laboratory adjudication in the vast majority of studies and different populations that were examined, but may also reflect the versatility of results that are markedly subject to appropriate patient selection, sizing, and positioning2.
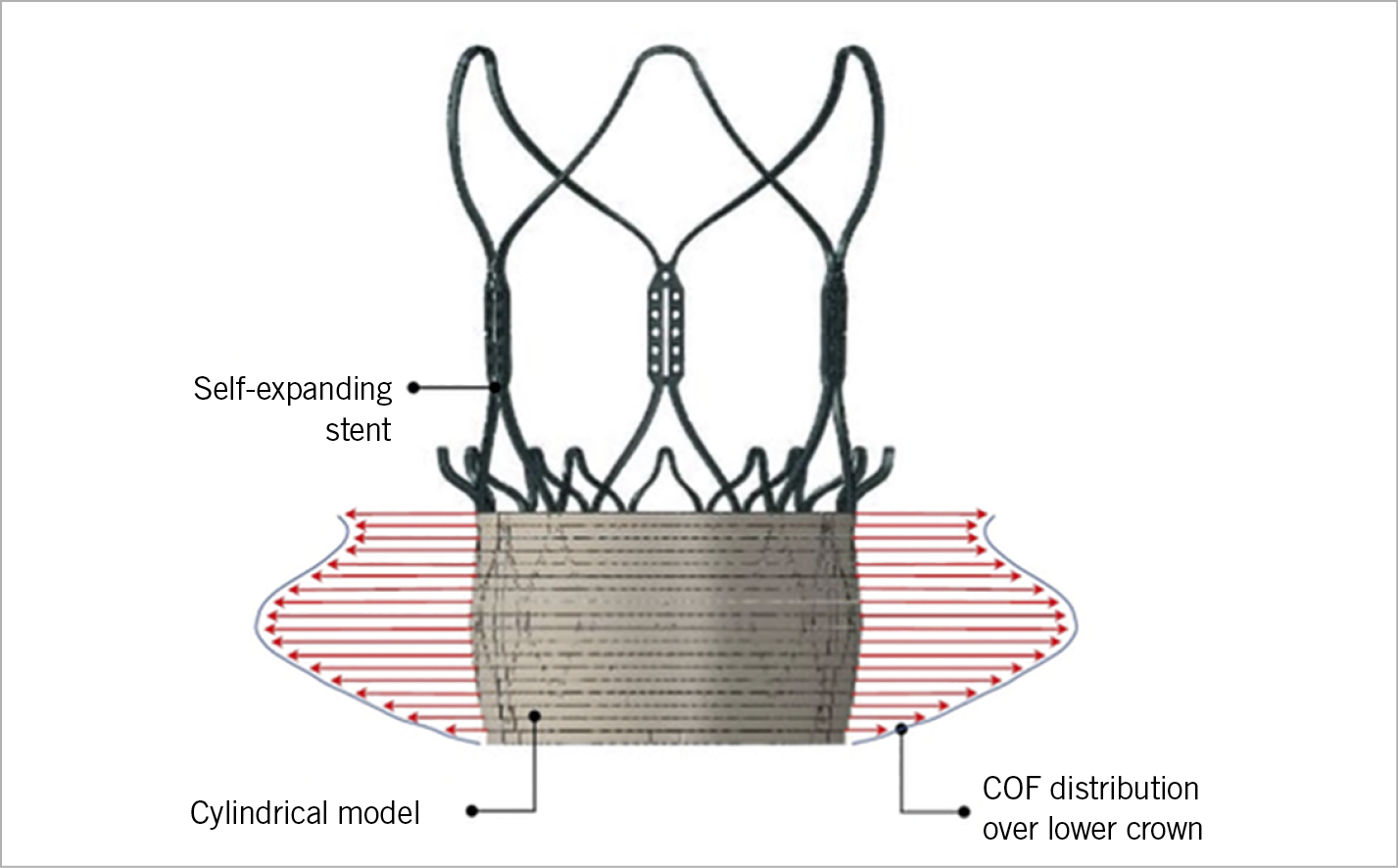
Figure 2. Radial force of the ACURATE neo. Distribution of the radial force (chronic outward force [COF]) over the height of the lower crown in contact with a cylindrical and compliant annulus model as estimated with the finite element method. The maximum force is located at approximately the mid height of the stent body.
There are several ongoing clinical studies that may corroborate existing data and fill knowledge gaps. Among these, the SCOPE II randomised trials for head-to-head comparisons of the ACURATE neo with the Evolut R/PRO platform, respectively, and the PROGRESS PVL registry for intra-individual, longitudinal assessment of the degree of PVL, should be mentioned. Enrolment has been completed recently for these studies and initial results will be available soon.
Patient selection
The ACURATE neo can be used in a wide clinical spectrum of patients, but there are some potential indications and anatomies where this device may be particularly suitable. Supplementary Table 1 provides an overview of the differential selection among commonly used TAVI prostheses.
SHORT CORONARY DISTANCE
The risk of coronary obstruction is relatively low since the upper crown keeps the native cusps away from the coronary ostia (Moving image 1). Accordingly, in the SAVI TF registry among 1,000 patients no case of coronary obstruction requiring intervention occurred4. Moreover, coronary re-access may be less challenging due to the short stent body and the open-cell design of the upper crown (Supplementary Figure 1).
SMALL AORTIC ANNULUS
The supra-annular design of the ACURATE neo translates into low mean transvalvular gradients, which may be of particular benefit for patients with small annuli to reduce the risk of PPM9.
HORIZONTAL AORTA AND TORTUOUS ANATOMIES
In horizontal aortic configurations, the short stent frame and the stabilisation arches provide a better coaxial alignment and thereby facilitate the deployment of the ACURATE neo. The flexible delivery system allows a smooth tracking of tortuous anatomies (Moving image 2, Moving image 3).
LOW PACEMAKER RATE
The rate of PPI is among the lowest for the ACURATE neo8,10,11, which may be attributed to the specific distribution of the moderate radial force (Figure 2) and less protrusion into the left ventricular outflow tract. However, recent data are not consistent and require further investigation6.
GENTLE PROCEDURE
The top-down release of the ACURATE neo without any need for rapid ventricular pacing allows haemodynamic stability throughout the entire implantation, as no outflow obstruction occurs during valve deployment. This may be beneficial in cases with impaired ventricular function or severe heart failure, particularly in cases where no predilatation and no post-dilatation are required12.
SEVERE AORTIC VALVE CALCIFICATION
As a caveat, due to its lower radial force, the ACURATE neo may be less appropriate in severe aortic valve calcification, where its use can result in higher rates of more-than-mild PVL and more frequent need for balloon post-dilatation2. However, Supplementary Figure 2 illustrates that the degree of PVL in severe aortic valve calcification depends not only on the total amount of aortic valve calcium, but also on its distribution.
BICUSPID AORTIC VALVE
The use of the ACURATE neo in bicuspid anatomies is feasible14. However, in the setting of very severe aortic valve calcification or asymmetric distribution, the use in bicuspid aortic valves may have an increased risk of device failure.
Sizing
The original sizing recommendation was adopted from the experience with the ACURATE TA bioprosthesis, which in fact differs from the ACURATE neo in many respects. Particularly in cases with borderline annulus dimensions, strict adherence to the official sizing chart may lead to relative undersizing. Supplementary Table 2 shows a modified sizing recommendation that was derived from a large, single-centre cohort to discriminate the risk of more-than-mild PVL2. In contrast to the official recommendation, annulus sizes below 21 mm can be treated without concern to a minimum of 19 mm, whereas the maximum size of 27 mm should not be exceeded, bearing in mind that the risk of residual PVL increases above an annulus size of 26.5 mm.
Procedural steps
A comprehensive overview of all relevant procedural steps for the implantation of the ACURATE neo is provided in Table 1, Moving image 4-Moving image 8, Figure 3-Figure 5 and Supplementary Figure 3-Supplementary Figure 5. Throughout the procedure, proper device positioning is key to achieving good results; once correctly positioned, upon full release the prosthesis will commonly stay within the intended landing zone due to predominant lateral extension and only minimal vertical motion.
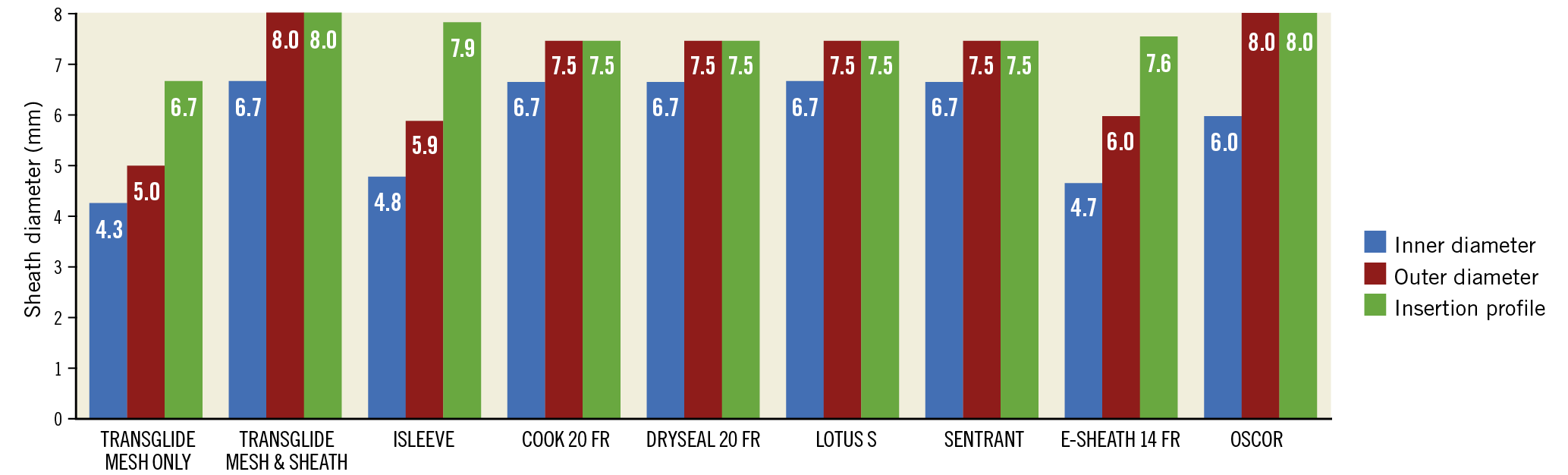
Figure 3. Introducer sheaths. The ACURATE neo system is compatible with various introducer sheaths that have different inner diameters, outer diameters, and insertion profiles.
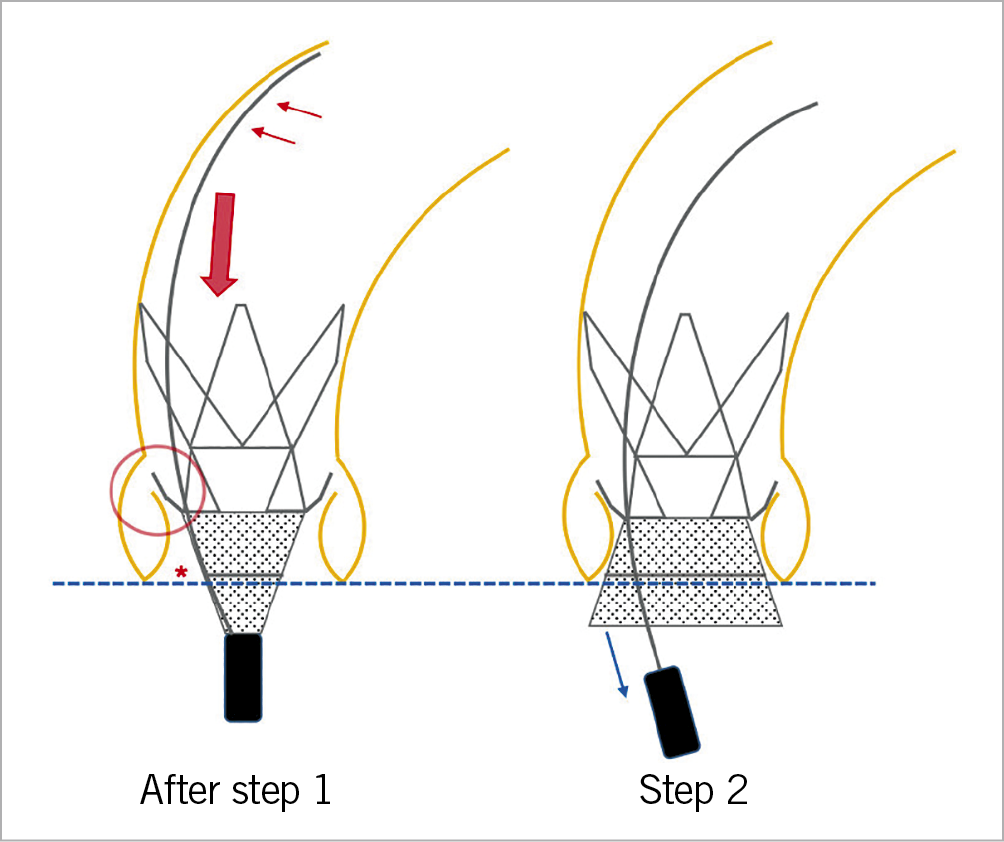
Figure 4. Positioning. The delivery system should be kept in the outer curvature (small red arrows) for enhanced stability during positioning. A proper device position is accomplished when the radiopaque intersection line (asterisk) is at the level of the annulus (dotted blue line), and the upper crown is in close proximity to the native leaflets (red circle). During step 2, an appropriate amount of forward tension should be maintained (large red arrow), trying to avoid too much (active) push on the device that might lead to ventricular embolisation.
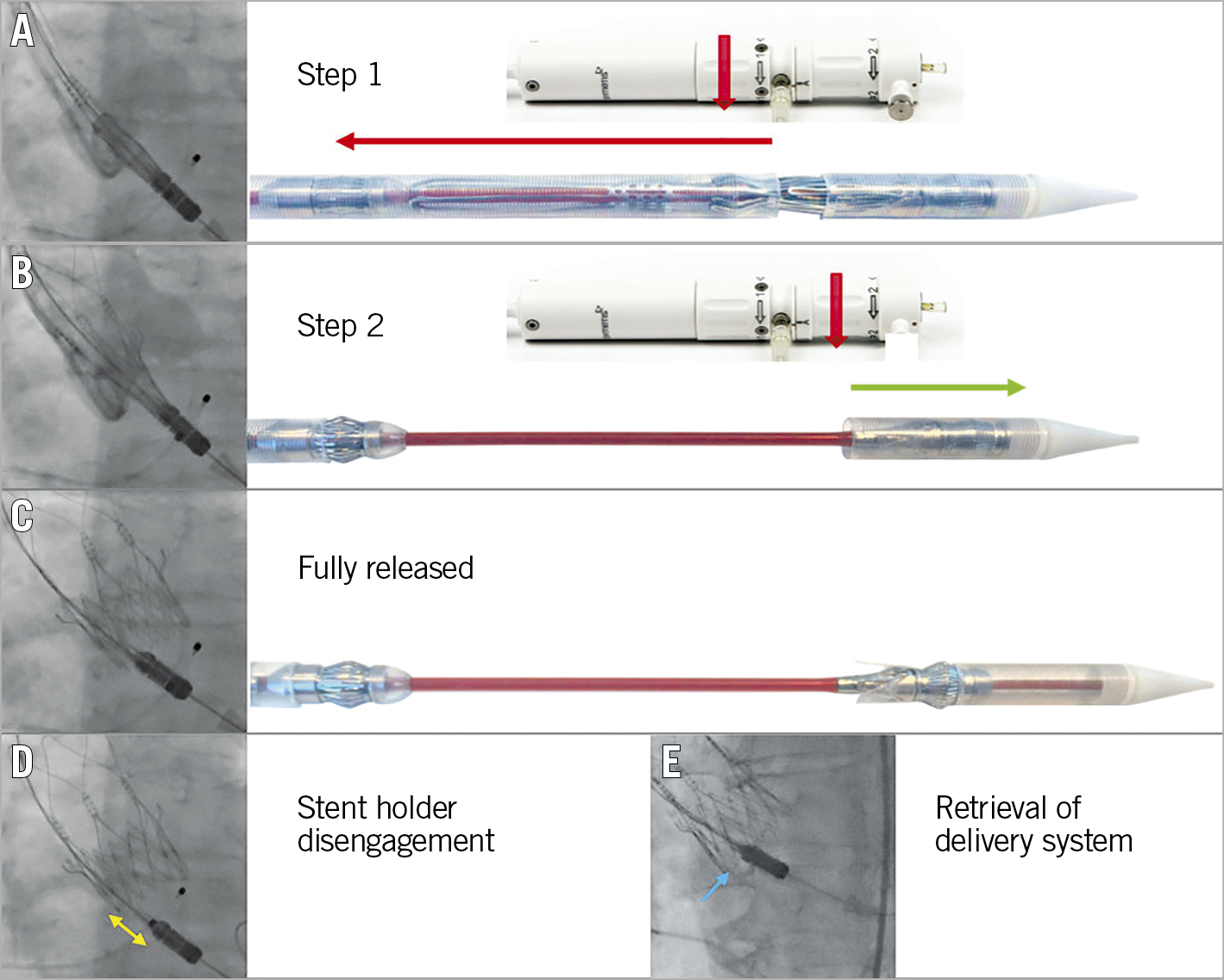
Figure 5. Deployment. After initial positioning of the prosthesis (A), turning the first rotation knob counter-clockwise releases the upper crown and the stabilisation arches (step 1). Thereafter, turning the second rotation knob counter-clockwise for step 2 (B) fully releases the prosthesis (C). D) Complete disengagement of the stent holder from the prosthesis (yellow double arrow). For retrieval of the delivery system out of the left ventricle, the guidewire should be pulled until the nose cone centralises (E).
Perspectives
1. The next-generation ACURATE neo2™ aortic valve system (Boston Scientific) has a dedicated sealing skirt that is designed to reduce PVL further, especially in the setting of heavily calcified annuli; the new system underwent initial clinical testing in 2018.
2. Implantation of the current version of the ACURATE neo is generally perceived as easy and intuitive. Less experienced operators would nonetheless be even more confident if valve repositioning or retrieval were possible.
3. The current version officially covers an annular size range from 21 to 27 mm. The smallest size is most probably also suitable for smaller annuli in an off-label fashion, whereas annuli >27 mm cannot be treated. Therefore, additional valve sizes, particularly for annulus dimensions above 27 mm, would further expand the spectrum of patients who can be treated with this device.
4. Further clinical data, especially from randomised trials, are awaited, as outlined above. This holds true especially for the evolving field of intermediate- to low-risk patients, since this subgroup was not enrolled in the initial ACURATE neo trials. Moreover, additional data will be important to clarify inconsistencies that were introduced by the most recent SCOPE I trial.
Conclusions
The worldwide increasing use of the ACURATE neo system is endorsed by a growing body of evidence. The optimal distribution of the relatively moderate radial force and its unique principle of deployment account for its notably balanced profile. Importantly, careful patient selection, proper sizing, and appropriate positioning are premises for optimised outcomes.
Appendix. Study collaborators
Mirko Doss, MD, PhD; Department of Cardiac Surgery, Kerckhoff Heart Center, Bad Nauheim, Germany. Andreas Holzamer, MD; Department of Cardiothoracic Surgery, University Medical Center, Regensburg, Germany. Oliver Husser, MD, PhD; Department of Cardiology, St. Johannes Hospital, Dortmund, Germany. Cindy Belon; Boston Scientific, Ecublens, Switzerland. Stefane Delaloye, PhD; Boston Scientific, Ecublens, Switzerland.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Acknowledgements
We are grateful for the help of Elizabeth Martinson, PhD, of the KHFI Editorial Office, in preparing the manuscript.
Conflict of interest statement
W. Kim declares proctor fees from Boston Scientific and Abbott, and speaker fees from Boston Scientific, Abbott, Medtronic, and Edwards Lifesciences. C. Hengstenberg declares proctor and speaker fees from Boston Scientific. M. Hilker declares proctor and speaker fees from Boston Scientific. U. Schäfer is a consultant and proctor for, and is on the speaker’s bureau for Abbott Vascular, Boston Scientific, Edwards Lifesciences, Medtronic, Gore, JenaValve, and New Valve Technology, and has received research grants from Abbott Vascular, Boston Scientific, JenaValve, Edwards Lifesciences, and New Valve Technology. T. Rudolph is a proctor and/or has received speaker honoraria from Boston Scientific, JenaValve, Edwards, Medtronic, and Abbott. S. Toggweiler is a proctor and consultant for and/or has received speaker honoraria from Boston Scientific, New Valve Technology, Edwards Lifesciences and Medtronic, and has received a research grant from Boston Scientific. A. Rück declares being a proctor for Boston Scientific. O. Husser has received proctor fees from Boston Scientific. A. Holzamer has received proctor fees from Boston Scientific. L. Conradi declares being a proctor for Boston Scientific. M. Doss has received proctor fees and/or speaker honoraria from Boston Scientific, and Abbott. H. Möllmann has received proctor fees and/or speaker honoraria from Boston Scientific, Abbott, Biotronik, and Edwards Lifesciences. L. Søndergaard has received consultant fees and institutional research grants from Boston Scientific. C. Hamm reports being on the advisory board of Medtronic. C. Belon is an employee of Boston Scientific. S. Delaloye is an employee of Boston Scientific. The other author has no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. The upper crown keeps the native calcified cusp away from the left main ostium.
Moving image 2. Severe aortic tortuosity and horizontal aorta.
Moving image 3. Smooth advancement of the ACURATE delivery system through the tortuous aorta and across the aortic arch.
Moving image 4. Appropriate forward pressure on the delivery system during step 2.
Moving image 5. Inappropriate push on the delivery system leading to ventricular embolisation.
Moving image 6. Aortic migration of the prosthesis during balloon retrieval after re-crossing through one of the stabilisation arches.
Moving image 7. After re-crossing of the prosthesis, wire manipulation reveals a slight inward bending of the adjacent stabilisation arch.
Moving image 8. Correct re-crossing of the prosthesis.

