Description - technical specifications
The success of the innovative Symetis ACURATE TA™ aortic bioprosthesis triggered the development of a new TAVI device, in which the specific features are translated for a transfemoral approach. The ACURATE TF™ aortic bioprosthesis (Symetis SA, Ecublens, Switzerland) is a self-expanding nitinol device, which was designed: 1) to allow easy and intuitive implantation; and 2) to overcome high rates of post-interventional pacemaker implantation and paravalvular leakage. Today, it is available in three sizes accommodating aortic annulus sizes from 21 to 27 mm (ACURATE TF™ S 21-23 mm, M 23-25 mm, L 25-27 mm). Several unique features characterise the stent architecture (Figure 1): 1) flexible stabilisation arches that are responsible for the self-aligning properties of the valve ensuring predictable coaxial alignment; 2) upper crown, which guarantees stable positioning and supra-annular anchoring of the valve capping the native leaflets and mitigating the risk of coronary obstruction and paravalvular leak; and 3) lower crown which opens upon full deployment and protrudes only minimally towards the left ventricular outflow tract.
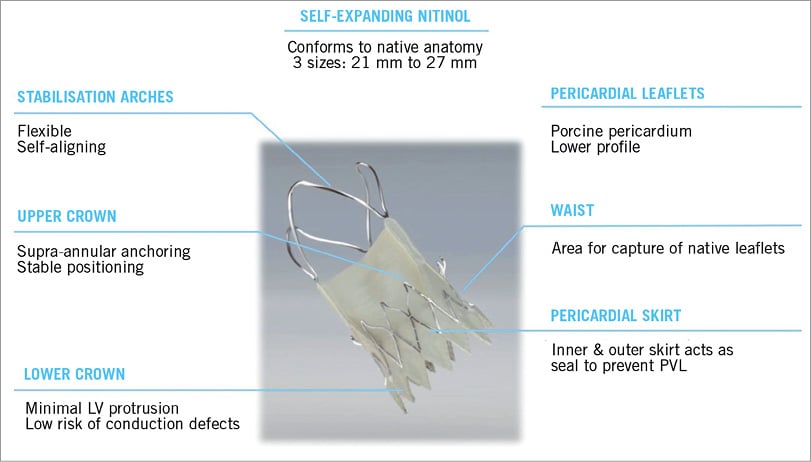
Figure 1. Characteristics of the ACURATE TF™ bioprosthesis.
The leaflets of the valve are made out of porcine pericardium. The valve leaflets are placed supra-annularly yielding low gradients. Importantly, the stent body and lower crown have a pericardial skirt on the interior and exterior of the stent, which acts as a seal preventing paravalvular leak.
The ACURATE TF™ delivery system has a very flexible shaft facilitating easy tracking even in tortuous aortic anatomy. It allows a controlled three-step implantation of the prosthesis (Figure 2).
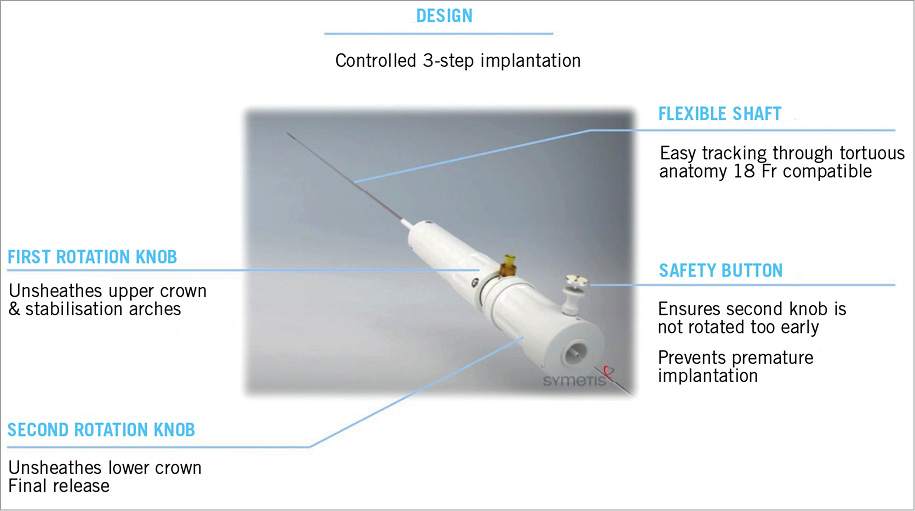
Figure 2. Characteristics of the ACURATE TF™ delivery system.
Implantation procedure
Access to the femoral artery, retrograde passage of the aortic valve and secure placement of a super stiff guidewire are established according to standard procedures. At present a 20 Fr insertion sheath should be used. However, the technical development of a true 18 Fr system has successfully been accomplished and been filed for approval. A balloon valvuloplasty prior to valve implantation is not mandatory but strongly recommended. The implantation should be performed with a perpendicular view on the annulus. The ACURATE TF™ prosthesis can easily be advanced through the aortic arch. It is primarily designed for a two-operator procedure with the first operator controlling the exact position of the valve and the second operator being responsible for the stepwise release. However, the entire procedure can also –in experienced hands– be performed by a single operator.
The valve has initially to be aligned 2-4 mm within the annular plane (Figure 3). Unlike other self-expanding devices, the Symetis ACURATE TF™ is not released from the ventricle to the aorta but rather from the top down, something which adds to the system’s stability. The implantation of the valve is an intuitive controlled three-step procedure:
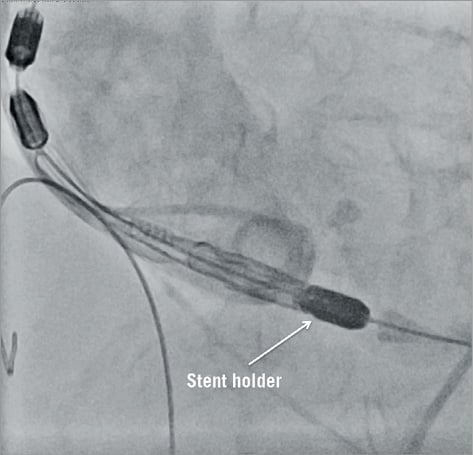
Figure 3. Placement of the ACURATE TF™ bioprosthesis.
STEP 1
The first rotation knob is turned counter-clockwise in order to unsheath the upper crown. The deployed upper crown can then be used to capture the native leaflets and push them down gently. The desired implantation depth is 4-6 mm below the annulus (Figure 4).
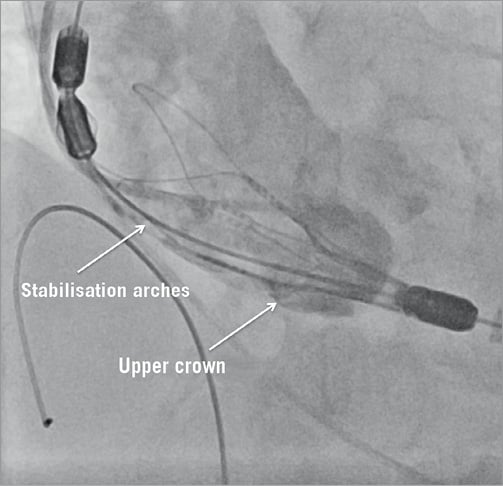
Figure 4. Stepwise release of the bioprosthesis.
STEP 2
The opening of the stabilisation arches is performed by further counter-clockwise turning of the first rotation knob. These two steps do not compromise the aortic outflow. After opening the stabilisation arches the proper placement of the valve in relation to the native annulus should be checked once more (Figure 4).
STEP 3
Final deployment of the valve can only be performed once the safety button has been removed. Unsheathing of the lower crown should be performed under a short episode of rapid ventricular pacing. The valve is released through counter-clockwise turning on the second rotation knob. After full deployment, the delivery system can be carefully retrieved (Figure 5).
Figure 5. Final result.
Clinical data
The first-in-human use was performed in January 2012 in three patients. These patients have returned for a 12-month follow-up in good health and good function of the device without significant residual stenosis or paravalvular leakage. The first-in-man trial (TF20) enrolled 20 patients in one centre in Brazil and three centres in Germany between February and August 2012. The next trial (TF50) is currently enrolling.
In the TF20 trial, patients were 84.8±4.5 years old and presented with a logistic EuroSCORE of 26.5±8.0 (STS score 7.0±5.2). The intended implantation could be performed in all patients. The procedural success was very high at 95% (n=19); only one patient had to be treated with a valve-in-valve due to too low initial placement. One patient had a grade 2 paravalvular leak, while all other patients had zero or trace leaks. The effective orifice area improved from 0.7 cm2 to 1.8 cm2. The pacemaker rate was low at 10% (n=2). At 30 days, there was one stroke and one reintervention (due to a VSD at day 17). Importantly, there was no myocardial infarction and no death at 30 days of follow-up. Likewise, there was a significant improvement in NYHA class in all patients.
Discussion
The number of high-risk patients undergoing percutaneous valve implantations is still increasing. However, most procedures are still performed with the first-generation devices SAPIEN (Edwards Lifesciences, Irvine, CA, USA) and CoreValve® (Medtronic, Minneapolis, MN, USA) with the known shortcomings. Firstly, residual paravalvular leaks >1+ have been identified as an independent risk factor for mortality1. Secondly, the need for pacemaker implantations varies between different systems from 10 to 25%2. Finally, the implantation process is not always easy. These points have been addressed by second-generation percutaneous bioprostheses.
The transapical Symetis ACURATE TA™ received CE mark approval in September 2011. This bioprosthesis proved to achieve very good haemodynamic results in combination with low pacemaker rates3,4. The aim of developing the ACURATE TF™ bioprosthesis was to translate the key features of this valve to a transfemoral platform. The first-in-human-use patients returned in the meanwhile with very good 12-month results. The results of the first-in-man patients (TF20) were likewise very promising. The rate of paravalvular leaks is very low, as is the need for new pacemakers. The implantation process and especially the proper placement are easy.
The low rate of paravalvular leaks has already been described for the TA version of this bioprosthesis. There are two factors that might contribute to these promising results. First, the double layer skirt at the proximal part of the stent may help sealing against paravalvular leaks. Second is the specific implantation technique, where the upper crown is released slightly above the calcified leaflets and then pushed down, thus trapping and compressing the calcified material above the annulus.
The low rate of pacemaker implantation (10%) is remarkable given the self-expanding character of the ACURATE TF™, especially in the light of experience gained with other self-expanding devices. Again, there are two potential reasons to be discussed. Firstly, the bioprosthesis can be reliably and reproducibly placed rather high with only a few millimetres protruding in the left ventricular outflow tract. Secondly, the degree of radial force is rather low.
Last, but not least, the implantation process is easy to perform with an intuitive stepwise approach. During the first two steps the position can easily be corrected in the direction of the left ventricle. During final release no further correction of the valve’s position is necessary. These characteristics may be especially helpful since more and more centres are starting a TAVI programme, although it can be anticipated that only rather small numbers will be treated. Patients treated in these centres may therefore profit from a straightforward system not demanding numerous steps to remember.
There are several limitations to be noted. The delivery system requires a rather large sheath (currently 20 Fr, though an 18 Fr system awaits approval). The system does not allow resheathing, repositioning or retrieval once fully deployed and the architecture of the stent does not make it the first choice for valve-in-valve procedures.
Conclusion
The Symetis ACURATE TF™ aortic bioprosthesis has been designed for an easy and intuitive implantation. The first-in-man trial shows very promising results in terms of low rates of paravalvular leak and minimal need for pacemaker implantation. A larger cohort is currently under investigation, leading finally to an application for CE mark approval.
Conflict of interest statement
H. Möllmann is a proctor for Symetis, St. Jude Medical, and Medtronic, and has received lecture fees from St. Jude Medical, Medtronic, Edwards Lifesciences, and Abbott. P. Diemert has research contracts from and is a proctor for Symetis and Edwards Lifesciences, and has received lecture fees from JenaValve. E. Grube is on the scientific advisory board of Boston Scientific, Cordis Johnson&Johnson, Abbott, and Edwards Lifesciences, and is a proctor for Medtronic. S. Baldus has received lecture fees from Symetis. J. Kempfert is a proctor for Symetis. A. Abizaid has received a research grant from Symetis.
Online data supplement
Moving image 1. Placement of the ACURATE TF™ bioprosthesis.
Moving image 2. Stepwise release of the bioprosthesis.
Moving image 3. Final result.

