
Computed tomography (CT) is the standard of care for device sizing for balloon-expandable (BE) and self-expanding (SE) transcatheter aortic valve implantation (TAVI) and has dramatically impacted outcomes in this field, particularly the frequency of paravalvular aortic regurgitation (PAR)1,2. In this issue of EuroIntervention, Kim et al describe multicentric outcomes of 198 patients treated with a novel SE transcatheter heart valve (THV) device, the Edwards CENTERA (Edwards Lifesciences, Irvine, CA, USA) in the context of CT-based oversizing, as assessed prospectively by a dedicated CT core lab3.
The Edwards CENTERA is a relatively novel THV whose first-in-man (FIM) experience was described in 2010, but which has only recently completed a CE mark study4 and has yet to commence a US feasibility or pivotal study. The THV consists of a relatively short (in comparison to existing SE designs), contour-shaped, SE nitinol valve frame with bovine pericardial tissue leaflets. It is inserted by transfemoral access using an expandable 14 Fr introducer sheath and features a steerable delivery catheter to traverse the aortic arch and for coaxial alignment within the annulus, a loading capsule containing the pre-attached THV, and a tapered tip to facilitate valve crossing; a 6-V battery-powered motorised handle enables valve loading and deployment, as well as repositioning with the possibility of recapturing up to 85% of the deployed valve4.
Cardiac CT annular perimeter sizing was performed at the discretion of the site with baseline guidance of optimal oversizing in the 10% to 20% range. Importantly, predilatation was performed in all cases and post-dilatation in one third. Favourable outcomes were achieved overall with this approach to sizing with low rates of new permanent pacemaker implantation (PPI) and PAR and low transvalvular gradients with high effective orifice areas (EOA). Each of these important outcomes are related to THV sizing but are multifactorial and also related to several other factors that would have been difficult to comprehensively address in the present manuscript.
PERMANENT PACEMAKER
The rate of PPI was only 4.7% (nine cases), 5.5% for pacemaker-naïve patients, extremely low for an SE THV. This number of cases makes it challenging to reliably investigate its association with the extent of oversizing or other potential predictors. The potential variation in pacemaker indication across participating centres may also be relevant. According to the original report which described the 30-day results of the Safety and Performance Study of the Edwards CENTERA-EU study4, third-degree atrioventricular block (AVB) occurred in 14 patients, but only nine of them received new PPI. Moreover, the frequency of PPI must be interpreted in the context of pre-existing conduction disease in the form of right bundle branch block (RBBB), data that were not available in this focused CT-sizing paper.
PARAVALVULAR AORTIC REGURGITATION
PAR ≥mild was seen in 37.9% (with no cases of severe PAR and only 0.5% of PAR ≥moderate). This compares very favourably to both BE and contemporary SE TAVI data. However, in an additional three patients (1.5%), the THV was not successfully placed due to valve embolisation into the left ventricle in one patient and valve migration in one patient, with conversion to SAVR in both cases, as well as poor coaxiality in one case with subsequent implantation of a SAPIEN 3 THV.
THE RELEVANCE OF DEPTH OF IMPLANTATION
Although sizing for the BE SAPIEN 3 is relatively simple due to its cylindrical structure, self-expanding aortic THVs are contoured and non-cylindrical; this means that the interaction between device and annulus by degree of oversizing has a greater dependence on device positioning, data which are notably omitted from this paper and the original CENTERA CE mark study manuscript3,4. Depth of implant is also a critical determinant of several outcomes after both BE and SE TAVI, including PPI and PAR5,6. There are also historical data for the CoreValve® (Medtronic, Minneapolis, MN, USA) with higher depth of implantation improving effective orifice area (EOA) and prosthesis-patient mismatch7.
The shorter design of the CENTERA frame combined with an aggressively demarcated waist are unique features for an SE THV (Table 1) that may mandate and help facilitate a more standardised depth of implantation but means that leaflets are intra-annular. In contrast, the longer frame of the Evolut™ R/PRO (Medtronic), although permitting supra-annular leaflets and a greater degree of freedom in depth of implantation, may also allow a greater degree of variability in this parameter. Perhaps for this reason, a wide range of outcomes has been reported. Although instructions for use (IFU) for the Evolut R/PRO specify an implant depth of 3-5 mm, many operators, ourselves included, strive to routinely implant much higher at 1-3 mm. Interestingly, although SE THV data have generally reported much higher PPI rates than the CENTERA data, the ACURATE neo™ device (Boston Scientific, Marlborough, MA, USA) that incorporates technology to standardise depth of implantation (Table 1) has a low reported rate of new PPI of 8%8. Both device technology and operator technique demand focus on implanting SE THV as high (aortic) as possible in the aortic annulus to mitigate PPI.
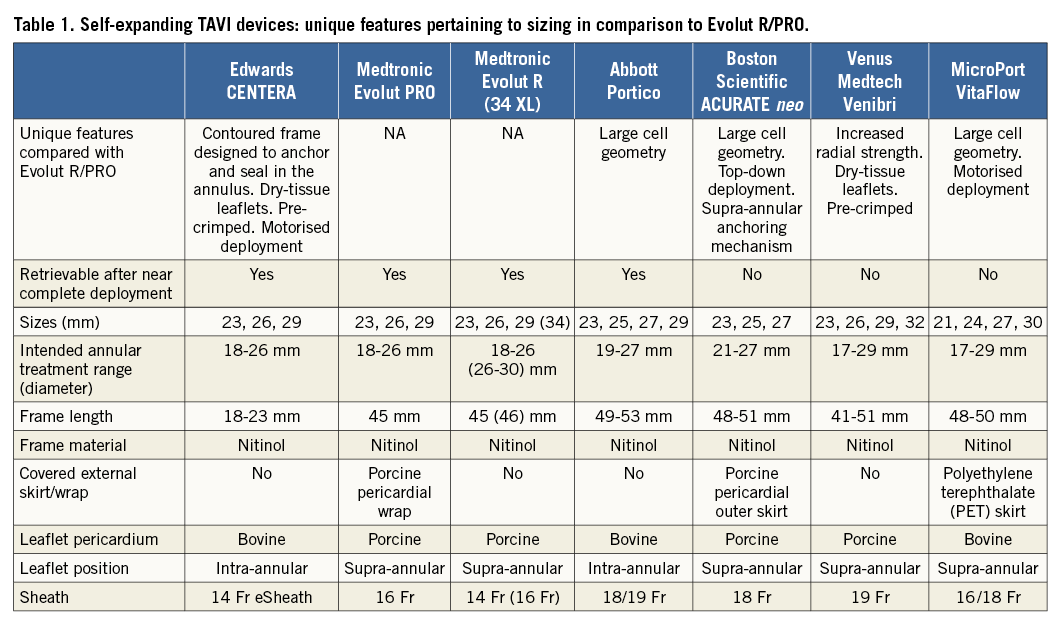
OVERSIZING, CENTERA AND OTHER SE TAVI DEVICES
The authors place considerable emphasis on higher rates of PPI and the greater influence of oversizing in SE TAVI devices other than the CENTERA. In this context, we examined data from our single-centre real-world experience of 175 consecutive patients undergoing TAVI between April 2017 and March 2018 with the latest iteration Medtronic device (Evolut PRO) which treats a similar range of aortic annular dimensions to the CENTERA (Figure 1). CT data were measured prospectively by a dedicated CT core lab; pre-discharge echo parameters were reported clinically according to VARC-2 criteria by four dedicated valve centre expert echocardiographers. With the Evolut PRO, we had a mean implant depth of 3.0±1.9 mm, PAR ≥mild of 29.9%, PAR ≥moderate of 0.6% and an EOA of 2.35±0.45 cm2 (Figure 1); excluding patients with prior PPI, new PPI was 5.7% (6.6% for pacemaker-naïve patients) at 30 days. Predilatation was performed in 27.0% and post-dilatation in 43.1%. In the setting of a systematically high depth of implantation and generally oversizing >10% (96% of cases) by perimeter (mean oversizing 17.6±5.1%), we saw a slightly greater influence of degree of oversizing and outcomes with our Evolut PRO experience than the CENTERA-EU data, but similarly low rates of PPI and favourable haemodynamics on echocardiography (Figure 1).
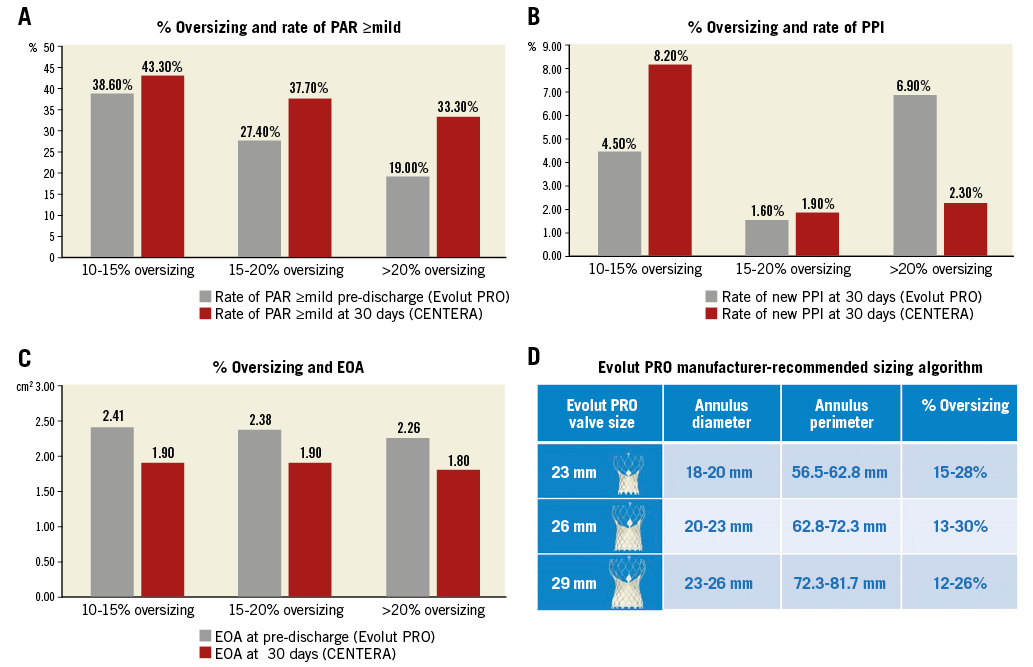
Figure 1. Comparison of oversizing in NYU Langone data with Evolut PRO (grey) with CENTERA-EU (red). Comparison of endpoints is shown for oversizing categories according to perimeter: A) Paravalvular regurgitation (PAR). B) Rate of permanent pacemaker implantation (PPI). C) Mean effective orifice area (EOA). To place % oversizing in context, the manufacturer-recommended Evolut PRO sizing table is shown with degree of oversizing for each valve size (D).
CALCIUM AND OVERSIZING
Aside from device sizing and depth of implantation, left ventricular outflow tract (LVOT) and aortic valve calcium have been identified as independent predictors of PAR and PPI in numerous studies of both BE and SE TAVI6,9,10, but not in the present study. Although data were not presented, it is conceivable that the extremes of aortic valve and subannular calcium severity may have been less frequently included in the setting of a clinical trial, where stricter anatomical selection criteria are generally adopted in comparison to a real-world experience. It is possible that the correlation of oversizing ratio and the incidence of PAR with the CENTERA could be potentially modified by aortic valve calcium severity and this merits further study in a real-world experience.
Conclusions
Although the data for the CENTERA device suggest a less dramatic interaction between degree of oversizing and THV outcomes than data previously published for other SE devices, this must be viewed in the context of depth of implantation and a systematic degree of oversizing ≥10%. Taking into account these factors, the favourable outcomes achieved with this device may also be achieved with other SE THV devices but may be more operator-dependent.
Conflict of interest statement
H. Jilaihawi is a consultant to Edwards Lifesciences and Venus Medtech and receives research funding from Edwards Lifesciences, Medtronic and Abbott Vascular. M. Williams is a consultant to Medtronic and Abbott Vascular and receives research funding from LivaNova, Medtronic, Bracco, Edwards Lifesciences, NeoChord, Abbott Vascular. Z. Zhao has no conflicts of interest to declare.

