
Abstract
Aims: The aim of this study was to introduce and demonstrate the feasibility in an acute preclinical model of a new transcatheter heart valve concept with a self-expanding wire frame, polymeric leaflets and a sealing component.
Methods and results: The TRISKELE valve was developed based on a previously validated polymeric leaflet design, an adaptive sealing cuff and a novel nitinol wire frame which reduces stress on the leaflets and radial pressure on the surrounding tissue. A valve prototype of 26 mm nominal diameter was manufactured by automated dip coating of a biostable polymer. The prototype was implanted via brachiocephalic approach in orthotopic position in an acute ovine model through a highly controllable multistage deployment process. The atraumatic retrievability of the valve after full expansion was verified in situ before final release in the optimal position. Observation indicated secure valve anchoring, adequate leaflet motion, and no interference of coronary flow or mitral valve function.
Conclusions: The TRISKELE valve system has the potential to mitigate complications related to imprecise valve positioning, and may offer a safer and more economical TAVI solution to a broad range of patients. The valve is currently under preclinical investigation for its long-term function and durability.
Abbreviations
Alpha-gal: galactose-alpha-1,3-galactose
EPC: endothelial progenitor cells
GLP: good laboratory practice
PCU: poly(carbonate-urea) urethane
POSS: polyhedral oligomeric silsesquioxanes
TAVI: transcatheter aortic valve implantation
UCL: University College London
Introduction
Transcatheter aortic valve implantation (TAVI) is rapidly expanding as the preferred alternative to surgery for the treatment of severe aortic stenosis in inoperable/high-risk patients1,2. Although current TAVI procedures provide remarkable benefits for elderly patients, they are associated with adverse events such as aortic regurgitation, atrioventricular block, difficulties in vascular access, peripheral embolism and risk of stroke1. The adoption of TAVI raises procedural challenges, including problems with secure and correct positioning of the prosthesis, risk of prosthesis migration, and complications related to valve-in-valve implantations. Paravalvular leak, in particular, is a major issue often occurring immediately after the valve deployment, due to prosthetic misplacement, incomplete valve deployment, or presence of heavily asymmetric calcifications in the aortic annulus1. The unknown durability of the TAVI prostheses is another concern which, although so far acceptable for elderly patients with limited life expectancy, needs to be verified (and most likely improved) before expanding the application of TAVI to the increasing number of patients with intermediate- and lower-risk profiles3. Cost is also a problem influencing the implementation of the TAVI therapy, which depends on device manufacturing costs as well as procedural and follow-up expenses4. The development of advanced technologies is therefore essential to address the need for safer, more affordable, and less complex TAVI devices.
In the present study, we introduce a new transcatheter heart valve concept recently developed at University College London (UCL), London, United Kingdom. The TRISKELE is a self-expanding valve with polymeric leaflets, intended to minimise procedural complications for high-risk patients and provide a solution for less risky patient demographics at a lower cost. This study aimed to evaluate the feasibility of the new delivery system and haemodynamic performance of the TRISKELE valve in an acute preclinical ovine model.
Methods
LEAFLET MATERIAL, DESIGN AND MANUFACTURING
The TRISKELE valve consists of three flexible leaflets adopted from a previously published novel polymeric valve design numerically optimised to have minimised energy absorbed during the operating cycle, resulting in improved hydrodynamic performance and reduced stress levels5. The trileaflet polymeric valve is supported by a self-expanding nitinol wire frame which stands 18 mm high and has a nominal diameter of 26 mm (Figure 1). The valve benefits from a flexible adaptive sealing cuff, made from the same polymer used for the leaflets, which conforms to the surfaces of the native anatomy and reduces periprosthetic regurgitation. The frame is obtained from a set of super elastic nitinol wires, thermomechanically shaped and joined by crimping sleeves. It provides multistage retrievability and repositionability of the valve with minimal stress on the collapsed frame during the loading/expanding phases, and maintains secure anchoring under conservative physiological pressure levels. The outflow portion of the wire frame features three lateral ribs defined by sets of smoothly arched petal-like shapes that protrude radially further than the flow-control structure. This unique geometrical feature helps maintain an open structure which reduces the impact on the surrounding tissue, also dampening the pressure load transferred to the leaflets while functioning. The ribs are curved to form small loops at the distal aortic region, enabling secure connection of a set of retrieval tethers that do not interfere with the functioning valve (Figure 1).
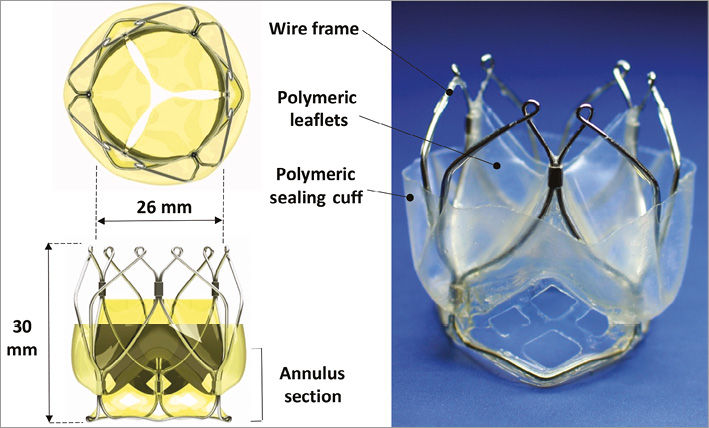
Figure 1. The TRISKELE valve with a 26 mm (nominal diameter) self-expanding nitinol wire frame loadable into a 20 Fr catheter. The valve consists of moulded nanocomposite leaflets and a sealing cuff, all formed by a one-piece membrane.
Polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU) was used to manufacture the leaflets and the sealing components of the TRISKELE valve. This is a nanocomposite polymer consisting of a hard crystalline segment and soft elastomeric segments in which polyhedral oligomeric silsesquioxane (POSS) nanoparticles are attached as pendant chain functional groups to the backbone of poly(carbonate-urea) urethane (PCU). Although it has never previously been used in heart valves, POSS-PCU has been validated in vitro for its enhanced haemocompatibility and thrombogenic-resistant surface6-8, biostability6, and resistance to calcification9.
The polymeric components of the TRISKELE valve, leaflets and sealing cuff were manufactured using an advanced automated manufacturing technique developed in-house, which allows constructing highly reproducible polymeric valves by robotic dip coating of a stainless steel mandrel into an 18% (w/v) polymer solution. This manufacturing approach is suitable for a wide range of biostable polymers that can be used in combination or as an alternative to POSS-PCU.
DELIVERY SYSTEM
A new delivery strategy was developed to simplify the loading, deployment and release of the valve while maintaining retrievability at any stage during the implant. In its current version, the delivery system comprises a loading/retraction system based on a set of closed-loop nylon threads (tethers) retractable axially along the sheath, a 20 Fr cover sheath (though smaller diameters are achievable), and a final release mechanism configured to constrain the tethers radially (Figure 2A). No loading system is required as the prosthesis can be loaded into the cover sheath by pulling and tightening the tethers, passed through the small loops of the valve frame, such that the distal (from the aorta) ends of the prosthesis are collected together and can be safely forced to collapse into the sheath (Figure 2B). The valve deployment is performed by turning the “sheath actuator” to unsheath and partially expose the valve and steer the catheter for optimal positioning (Figure 3A, Figure 3B). Once the optimal implant position has been reached, the valve can be fully expanded by adjusting the “control loops slider” to loosen and extend the tethers for about 1 cm (Figure 3C, Figure 3D). The valve is fully expanded and functional at this stage and can be examined for its haemodynamics, correct sizing, coronary interference, and migration (Figure 3D). Although functionally disconnected from the catheter and fully expanded, the prosthesis is still attached to the delivery system by the tethers. If necessary, the valve can still be retrieved at this time by reversing the deployment process to retract the control threads and resheath the prosthesis (Figure 2B). Once the position and haemodynamics of the valve are judged satisfactory, the valve can be released by pulling the “final release mechanism” to disengage the prosthesis from the tethers and the delivery system.
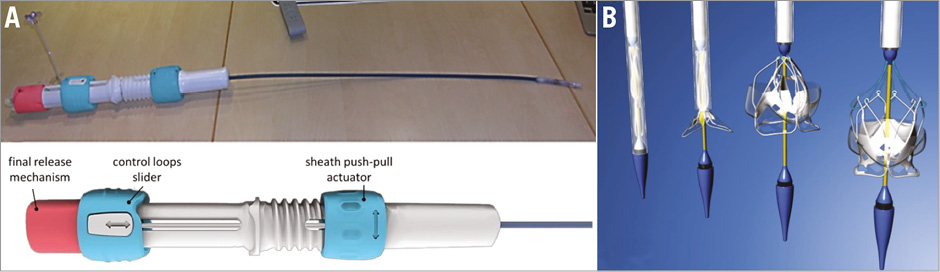
Figure 2. The TRISKELE valve delivery system. A) The control handle enables multistage loading and deployment of the valve, with the possibility of repositioning to fine-tune the final implant even after full deployment. The valve maintains full retrievability even after full expansion and can be completely removed from the implant site, if necessary. B) The deployment sequence can be reversed to retrieve the valve.
IMPLANTATION
The valve was implanted in a 63 kg adult sheep (19 months old) at a GLP-compliant preclinical research centre, IMMR, France, in accordance with the Animals (Scientific Procedures) Act 1986. The animal was evaluated preoperatively for its general health and its aortic annulus size measured as 23.5 mm using echocardiography. A size 26 TRISKELE valve was implanted off-pump via a brachiocephalic approach in the orthotopic position. Live fluoroscopy and angiography were used to monitor the deployment, retrieval, and repositioning of the valve. Transthoracic echocardiography was performed to assess the valve haemodynamics during a 30-minute postoperative follow-up. After completing the procedure, the animal was sacrificed humanely by intravenous overdose of barbiturate, and the heart was explanted and dissected. The valve was photographed from both inflow and outflow sides, and the gross appearance of the leaflets was inspected for any damage.
Results
The TRISKELE valve was successfully implanted in an acute preclinical ovine model with a 30-minute postoperative follow-up. The delivery system was easy to handle and allowed controlled deployment of the valve. The full atraumatic retrievability of the valve was verified by deploying, checking the haemodynamics, resheathing and repositioning the prosthesis before finally releasing it in the optimal position (Figure 3, Moving image 1).
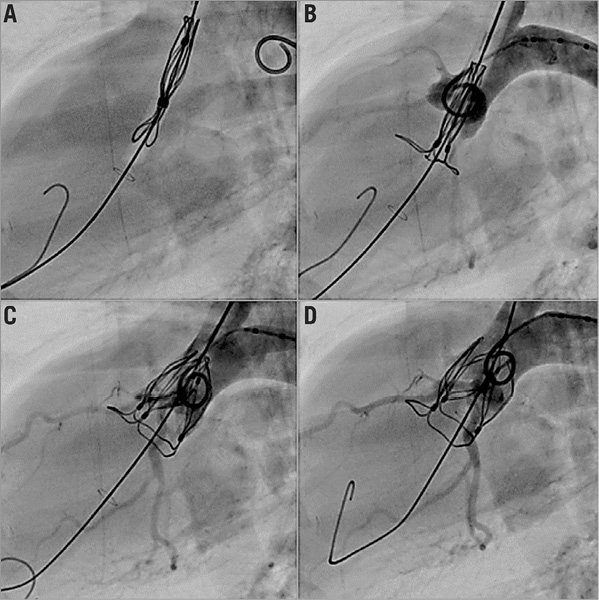
Figure 3. Live fluoroscopy illustrating the deployment of the TRISKELE valve. A) The valve advancing through the aorta. B) The inflow portion of the valve expanded into the ventricle and serving as a practical reference to find the optimum position. C) The valve being steadily deployed. D) Once the valve - fully functional at this stage - reaches the orthotopic region, the outflow protrusion ribs are expanded to perfect its alignment.
Prior to release of the valve, initial echocardiography revealed some intravalvular regurgitation, mainly due to the presence of the catheter (this disappeared after the removal of the delivery system), with mild paravalvular leakage. The valve maintained its anchoring after full release and removal of the catheter, functioning until euthanasia of the animal, administered after 30 minutes. The acute valve function demonstrated adequate leaflet motion (Figure 4, Moving image 2) and minimal flow obstruction (Figure 5, Moving image 3). The polymeric sealing cuff was found effective in minimising paravalvular leakage with some mild regurgitation, mainly due to the high compliance of the ovine aortic annulus. The valve haemodynamics were measured after the removal of the catheter, with a mean velocity of 785±159 mm/s, mean gradient of 3.0±1.3 mmHg, and an effective orifice area of 2.49±0.46 cm2. The peak velocity was measured as 1,068±275 mm/s and the peak gradient was 4.5±1.5 mmHg (n=3).
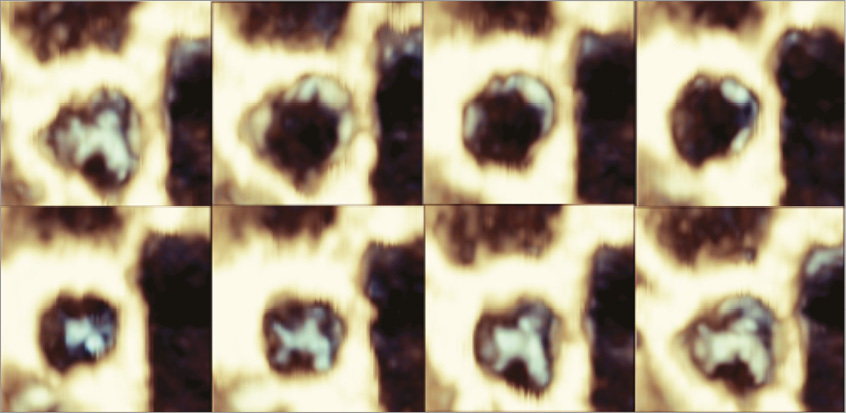
Figure 4. 3D echography reconstruction of TRISKELE leaflet motion over a cardiac cycle.
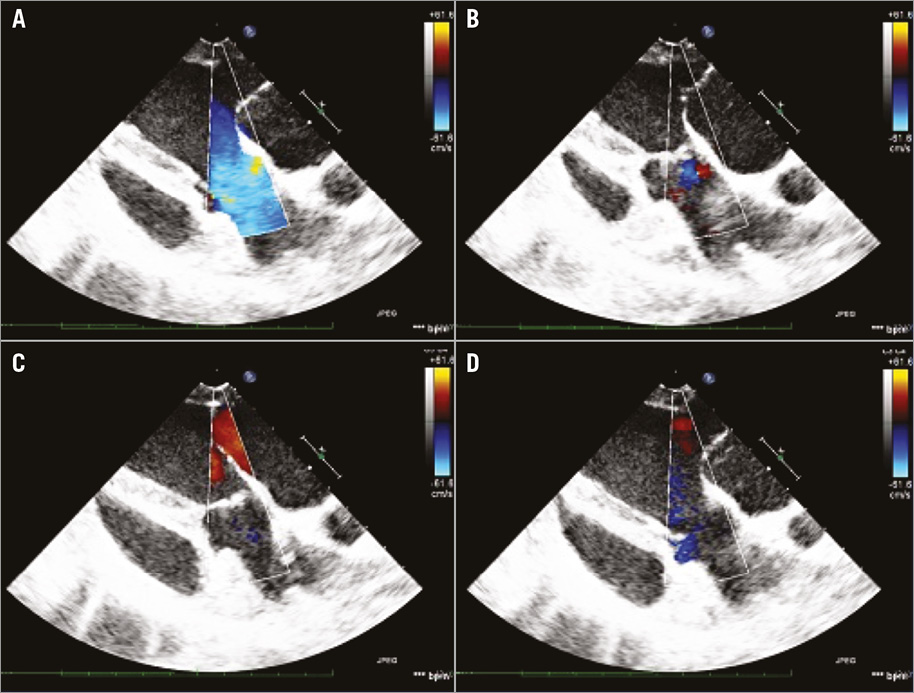
Figure 5. Echo-Doppler images of the TRISKELE valve. A) The systolic phase. B) The isovolumetric relaxation. C) The diastolic phase. D) The isovolumetric contraction over a cardiac cycle.
Post-implantation observation from the dissected heart showed that the TRISKELE had optimal alignment with the native anatomy (Figure 6). Once the valve was fully expanded, the three lateral ribs protruding from the outflow of the frame tended to sit in the sinuses of Valsalva, promoting a commissure-on-commissure alignment with the native valve. Observation also confirmed no interference with coronary blood flow or mitral valve function and a good matching with the anatomy of the left ventricular outflow tract.
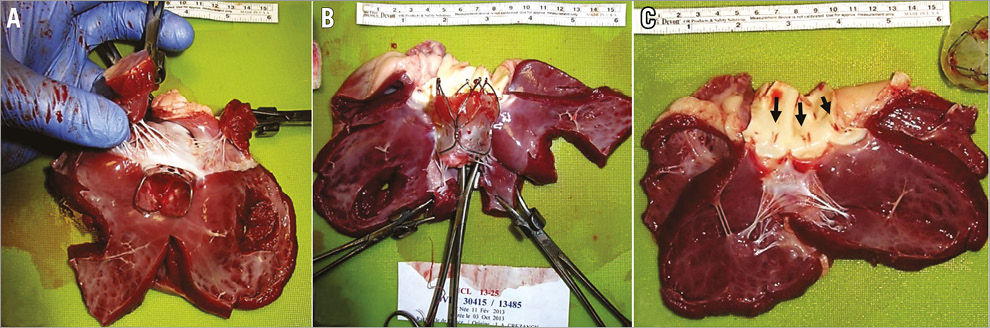
Figure 6. Dissection of the explanted heart after implantation of the valve. A) & B) No interference of coronary blood flow was observed. C) The TRISKELE valve was found to have aligned itself commissure-on-commissure with the native valve, owing to its outflow protrusions assisting optimal positioning. No sign of damage in the mitral valve.
Discussion
REPOSITIONABLE DESIGN
The TRISKELE valve system aims to minimise the complications related to imprecise valve positioning. It allows a highly controllable multistage deployment of the prosthesis. The valve starts functioning early in the expansion stage, which improves haemodynamic stability during deployment. It also maintains full retrievability at any time during positioning/repositioning and even after full expansion. The final implant configuration can be fine-tuned to correct possible imperfect seating of the valve and reduce any residual regurgitation. The valve remains connected to the delivery system (through the control tethers) until optimal position and haemodynamics are verified. If needed, the prosthesis can still be retrieved from the fully expanded state or completely removed from the implant site (prior to the final detachment of the control tethers) to accommodate any needed change of plan.
ANCHORING STRATEGY
The TRISKELE valve is secured in the implant site by applying mainly an axial clamping and minimum radial force. The wire frame forms an anchoring effect by exerting counteracting axial forces to the aortic annulus. The lower protrusions of the frame (inflow portion) project into the left ventricle and the upper petal-like ribs (outflow portion) expand radially on top of the valve annulus, into the leaflets of the native valve. The valve implantation is achieved by constraining the aortic annulus and not by dilating it, thus avoiding the application of high levels of distributed radial force which might perturb the atrioventricular node and the left bundle branch (Figure 7). This anchoring strategy also allows secure implantation of the TRISKELE valve in less calcific and less bulky anatomies of younger patients, as demonstrated in this study, where the valve was implanted in a highly compliant healthy native ovine aortic valve.
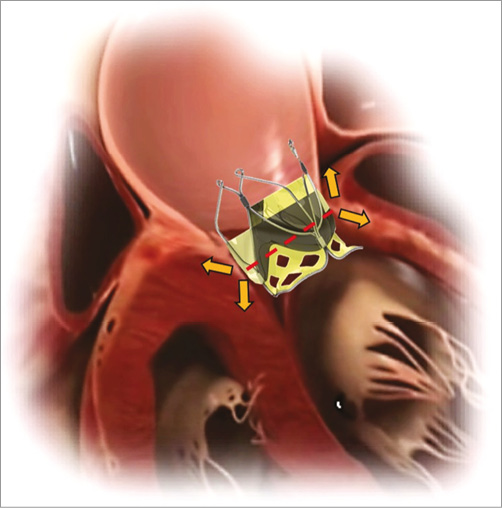
Figure 7. Anchoring of the TRISKELE valve. This is achieved by applying counteracting axial forces (clamping) to enhance sealing without applying excessive pressure on the annulus. This system wedges the valve in place, such that it cannot be displaced in either axial direction, avoiding the need for excessive radial force that increases the risk of atrioventricular block.
POLYMERIC LEAFLETS
Polymeric leaflets provide more design freedom compared to industry standard xenografts, and are particularly advantageous in transcatheter valve applications, enabling reduced leaflet thickness of about a third of that of pericardial leaflets. This helps achieve a thinner collapsible valve profile and minimise complications related to vascular access. The thinner polymeric leaflets also improve the haemodynamics of the valve, making it easier to open and achieve a larger orifice area. There is no suturing involved in the manufacturing process, hence no stitch hole in the leaflets which could cause tissue tear in the flexion zones.
Polymeric leaflets can address the concerns regarding tissue dehydration and leaflet damage believed to be experienced by current xenograft TAVI prostheses during the loading into the catheter10. Contrary to pericardial tissues which are prone to collagen fibre damage, polymeric leaflets are less susceptible to physical impairment induced as a result of collapsing/crimping. It is possible to preload the polymeric valve in air long before the operation, without causing any observable harm to the valve.
The Alpha-gal-free nature of biostable polymers means lower risks of calcification compared to porcine and bovine tissues, and may lead to potential advantages in terms of long-term performance and durability9. The other benefit of synthetic materials is their versatility. They can be engineered to demonstrate a tailored range of physical, chemical and biological characteristics. Advanced tissue engineering and bio-functionalisation techniques allow the creation of smart biomaterials with targeted behaviour, such as attracting endothelial progenitor cells (EPCs) and promoting in situ endothelialisation11.
COST BENEFITS
Polymeric valves are significantly cheaper and faster to construct, and can be manufactured with higher reproducibility compared to hand-made xenograft prostheses. Automated manufacturing can be utilised to mass-produce polymeric valves with more effective quality control arrangements. These valves are also easier and cheaper to store and sterilise. Furthermore, the availability of a preloaded off-the-shelf polymeric device potentially reduces the procedural time related to valve preparation/crimping, and can save costs. In the case of TRISKELE, the wire frame is obtained by thermomechanical shaping of nitinol wires, which is considerably cheaper compared to laser cutting processes used to manufacture most current TAVI devices. The TRISKELE delivery system is easy to use, highly controllable and reliable in deploying the prosthesis in the optimal position. This feature may be beneficial when performing so-called minimalist approach TAVI, with only a moderate sedation in a standard cardiac catheterisation laboratory and lower use of resources4, the success of which is highly dependent on a safe and accurate implantation of the prosthesis. These cost benefits may have a significant impact on the implementation of TAVI in developed countries with no need for operating theatres, and may also help to expand the application to developing countries.
Limitations
The main limitation of this study was the absence of calcification in the animal model and the relatively higher compliance of the ovine aortic annulus and root compared to humans. Moreover, transfemoral access would need the development of a smaller profile for the delivery system. Nevertheless, the successful implant under these conditions demonstrates that the TRISKELE valve may also be suitable for the treatment of valve insufficiency, expanding the selection criteria of patients suitable for TAVI.
Conclusions
An acute animal experiment was performed to demonstrate the feasibility of a new polymeric transcatheter heart valve. The TRISKELE valve was manufactured using a new automated technique which allows repeatable, rapid and cost-effective production of polymeric valves with superior reproducibility compared to hand-made xenograft prostheses. The valve requires minimum preparation and can be available preloaded on the catheter. The TRISKELE delivery system allows complete removal of the prosthesis from the implant site at any time during the procedure. The atraumatic retrievability of the TRISKELE was verified by fully expanding, checking the haemodynamics, resheathing and repositioning the valve before finally releasing it in the optimal position. The TRISKELE transcatheter valve offers a refined TAVI solution with potential impacts in terms of economic benefits and availability to a broader range of patients. The TRISKELE is currently under investigation in chronic animal experiments and accelerated wear tests.
| Impact on daily practice This study demonstrates the feasibility of the TRISKELE valve, a new transcatheter heart valve concept with a self-expanding nitinol wire frame, polymeric leaflets and a sealing cuff, which offers significant improvements to the current TAVI practice by providing a simpler and more reliable solution at a significantly lower cost. The TRISKELE delivery system allows a controllable deployment, repositioning, and atraumatic retrieval of the prosthesis even after complete expansion, with the possibility of ensuring a successful implant by verifying optimal position and haemodynamics of the valve prior to final release. The TRISKELE valve is significantly more economical to manufacture compared to xenograft devices, is available preloaded in the catheter and requires no preparation at the implant site. Having successfully completed feasibility implants in a non-calcific animal model with a relatively more compliant aortic annulus, the application of the TRISKELE valve system can potentially be expanded to a broader range of patient profiles. |
Acknowledgements
The authors would like to thank the research team at Institut Mutualiste Montsouris (IMM, Paris, France) for their assistance with animal studies, and also the National Institute for Health Research (NIHR, London, United Kingdom) for their support.
Funding
This study was supported by Wellcome Trust UK translational award 095747/Z/11/Z.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Fluoroscopic illustration of the TRISKELE valve implantation.
Moving image 2. 3D echography reconstruction of TRISKELE leaflet motion.
Moving image 3. Echo-Doppler haemodynamic assessment of the TRISKELE valve.
Supplementary data
To read the full content of this article, please download the PDF.
Fluoroscopic illustration of the TRISKELE valve implantation.
3D echography reconstruction of TRISKELE leaflet motion.
Echo-Doppler haemodynamic assessment of the TRISKELE valve.

