Abstract
Aims: Transcatheter aortic valve implantation (TAVI) is an emerging field with technological challenges. One major challenge is to minimise delivery catheter size to reduce vascular trauma, while maintaining features such as repositionability and retrievability. This study was designed as proof of concept of FOLDAVALVE, using a short-term ovine model.
Methods and results: FOLDAVALVE is a fully repositionable and retrievable transcatheter aortic valve with a 14 Fr delivery system whose leaflets are excluded from the stent during crimping. A short-term ovine model (n=3) was used for transfemoral implantations at the Institut Mutualiste Montsouris in Paris. There was a smooth transition of the valve over the aortic arch followed by seamless delivery into the native ovine aortic position. Implantation was followed by repositioning, resheathing and retrieval, then implantation of a fresh prosthesis in the same animal. Transoesophageal echocardiography and fluoroscopy were used to monitor the delivery and implantation processes and to demonstrate valve functionality. Animal sacrifice and direct visualisation after explant confirmed excellent final position.
Conclusions: FOLDAVALVE was successfully implanted in the aortic position of an ovine model. FOLDAVALVE is a promising technology which has been shown to be feasible in this preclinical TAVI study.
Abbreviations
AS: aortic stenosis
LV: left ventricle
PARTNER: Placement of AoRTic TranNscathetER valve trial
SURTAVI: Surgical Replacement and Transcatheter Aortic Valve Implantation
TAVI: transcatheter aortic valve implantation
TEE: transoesophageal echocardiogram
TTE: transthoracic echocardiogram
Introduction
Transcatheter therapies for severe aortic stenosis (AS) have progressed rapidly over the past few years1. Based on the success of the PARTNER trial and the CoreValve U.S. Pivotal trial, multiple valves are in use for patients at high or extreme surgical risk1-3. Trials such as PARTNER II and SURTAVI are underway to evaluate the efficacy and risks of transcatheter aortic valve implantation (TAVI) in patients with intermediate surgical risk4,5.
Some of the most common complications of TAVI are major and minor vascular complications6, which can be due to a number of factors, including high sheath to femoral artery ratio7. This can be controlled, as the sheath to femoral artery ratio is entirely dependent on the device which is being used and its sheath size7. Another important factor to consider in valve design is durability8. Patients with an intermediate surgical risk tend to be younger, have fewer comorbid medical issues and thus a longer life expectancy8. Therefore, valve durability has become of paramount concern. Crimping has been shown to damage the microstructure of pericardial leaflets permanently9-12, and may lead to decreased durability, although this has not been definitively shown up to this point. In lower-profile valves, increased crimping induces more damage to the leaflets than larger devices. As the trend towards lower-profile devices continues, this damage should be somehow mitigated9. Finally, the ability to reposition a valve prior to final deployment and to retrieve it once it is deployed is critical due to the negative consequences of malpositioning.
FOLDAVALVE (FOLDA LLC, Rancho Santa Margarita, CA, USA) is a self-expanding 14 Fr transcatheter aortic valve (TAV) which is deliverable through the femoral artery (Figure 1, Figure 2). Due to its unique delivery system, INGENUITY (FOLDA LLC) (Figure 3), the valve is fully repositionable and retrievable. It utilises a novel mechanism to exclude the valve leaflets from the stent crimping process. Here we describe the preclinical assessment of FOLDAVALVE in a short-term ovine model.
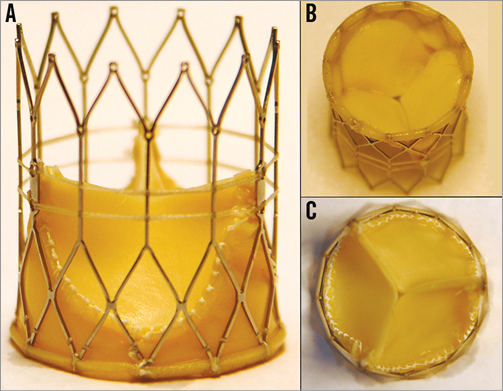
Figure 1. FOLDAVALVE in its expanded form. A) A lateral view. B) Ventricular side of the valve. C) Top down view of the aortic side. The distal end overlap of the valve extends over the expanded stent and functions to minimise paravalvular leak.
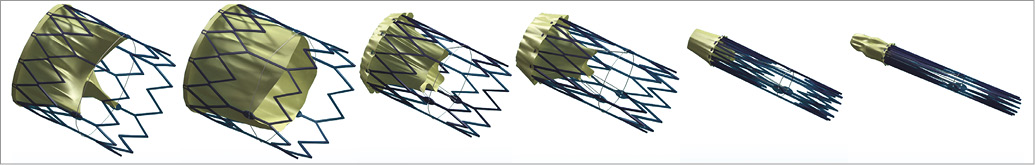
Figure 2. FOLDAVALVE transcatheter aortic valve. The 25 mm diameter frame can be collapsed to a 14 Fr delivery size, allowing for ease of access compared to larger TAV catheters. Here the process of reducing the profile of the valve can be seen from left to right. As can be seen on the right, the leaflets are excluded from the stent frame during crimping.
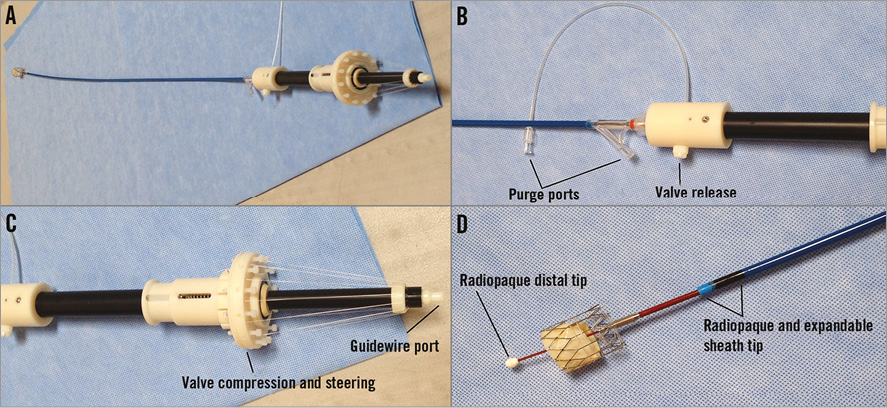
Figure 3. The INGENUITY delivery system. A) The INGENUITY system attached to FOLDAVALVE. B) The valve release and purge ports. C) The handle with its controlling force fibres which help repositioning, recapture and release of the valve. D) The distal end of the catheter and how FOLDAVALVE is attached to it.
Methods
VALVE CHARACTERISTICS
FOLDAVALVE is composed of a self-expanding nitinol stent and three bovine pericardial leaflets (Figure 1). When crimped, the leaflets are folded outside of the nitinol frame and are pulled into the expanding stent using a drawstring mechanism during deployment. Formation of the trileaflet valve occurs simultaneously with the stent expansion (Figure 2, Moving image 1). These unique design features allow the valve’s leaflets to be spared from damage that occurs during stent crimping and valve delivery9-12. During delivery and positioning, the valve is fully repositionable and retrievable through the INGENUITY delivery system. The INGENUITY system allows for six degrees of freedom in valve positioning and repositioning, and can be used for a femoral, subclavian or direct aortic approach (Figure 3, Moving image 2).
The radiopaque nitinol frame, in addition to markers on the delivery system, is used to guide implantation of the valve in the aortic position. Valve compression, steering and release are controlled by the operator to attain a satisfactory position for implantation (Figure 3). Repositioning occurs by partially compressing the stent back into the delivery system by means of force fibres which temporarily connect the stent to the delivery system. Compressing the stent to a size less than the native valve’s annulus allows for seamless repositioning of the valve in six degrees of freedom (Moving image 2). The retrieval process, which can be performed any time before the full release, is initiated by collapsing the stent with the leaflets inside by means of the force fibres. This results in complete resheathing and facilitates retrieval. Once fully resheathed, the valve is retrieved via the INGENUITY system and will not be used for reimplantation; a new valve system will be introduced to ensure the leaflet quality. At any time before full release and separation from the delivery system, the valve can be fully retrieved.
SHORT-TERM ANIMAL MODEL
To determine the feasibility and safety of FOLDAVALVE implantation, a short-term animal study was designed to test the capacity of the valve to be implanted, repositioned and retrieved. Three healthy domestic sheep (mean weight 45 kg) were used with a follow-up of 90 minutes post implantation. During the post-procedure monitoring, a complete transthoracic echocardiogram was obtained with haemodynamic assessment of the implanted valve. The animals were then euthanised after the 90-minute follow-up, and their hearts and aorta were harvested for gross macroscopic examination.
FOLDAVALVE IMPLANTATION
The animal protocols were developed in conjunction with and approved by the animal care and use committee of the Institut Mutualiste Montsouris (IMM), Paris, France. Transfemoral implantation of the FOLDAVALVE was performed by a multidisciplinary team. The procedure was undertaken in the hybrid operating room after pre-screening the animals for proper aortic root size.
Under general anaesthesia and mechanical ventilation, a 15 Fr introducer sheath was placed in one femoral artery for ease of manipulation. The INGENUITY delivery sheath was inserted into one femoral artery, while a standard 6 Fr introducer sheath was placed in the contralateral femoral artery. A 6 Fr pigtail catheter was then inserted and positioned in the aortic root. Following an aortic root angiogram, a 0.035” J-tipped guidewire was inserted and advanced into the LV after which it was exchanged for a stiff pre-shaped guidewire.
FOLDAVALVE was then loaded onto the delivery system and advanced into the LV, then pulled back to the aortic root (Figure 4). Under angiographic guidance, the valve was deployed, repositioned, properly positioned, and finally released (Figure 5, Moving image 3). After initial deployment, the valve was partially recaptured by the delivery system, repositioned and then redeployed into the native aortic valve followed by repeated assessment. The valve was then fully released (Moving image 3). In one of the cases, the fully deployed valve was recaptured and fully removed from the animal, and a new valve system was then introduced and implanted in the same animal to test FOLDAVALVE’s retrieval capacity (Moving image 4). A final evaluation was performed that consisted of angiographic, transthoracic echocardiographic (TTE) and haemodynamic evaluation of the FOLDAVALVE. Patency of the coronary arteries was assessed and prosthesis function was evaluated including valvular and paravalvular regurgitation. LV function was monitored and transvalvular gradients were measured.
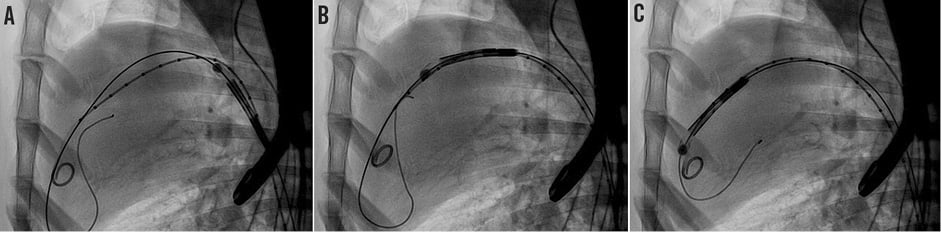
Figure 4. FOLDAVALVE being easily passed over the aortic arch and into the left ventricular cavity immediately before staged deployment and positioning in the aortic root. A) The valve passes up the descending thoracic aorta. B) This valve smoothly passes over the aortic arch and across the native aortic valve. C) The valve is being positioned from the left ventricle into the native aortic valve.
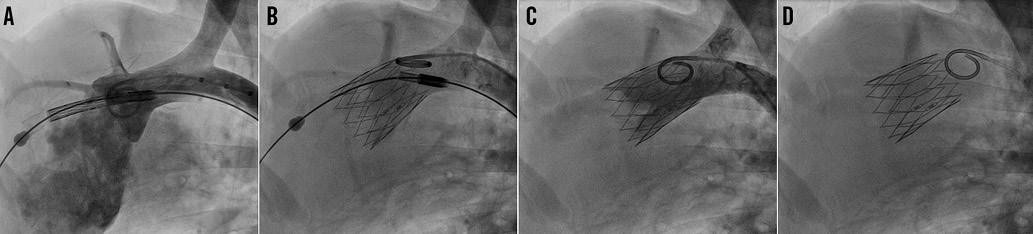
Figure 5. Positioning and release of the FOLDAVALVE. FOLDAVALVE is pulled back into the aortic root and positioned properly (A). The valve is released from the delivery system (B & C). The valve is fully expanded and functional at the aortic valve position (D).
Results
IMPLANTATION
FOLDAVALVES were successfully implanted in three domestic sheep under fluoroscopic and transoesophageal echocardiographic (TEE) guidance. The prostheses were easily able to traverse the aortic arch and be implanted in the aortic position (Figure 4, Figure 5). After initial implantation, the valves were partially recaptured then repositioned and reimplanted. Retrieval, repositioning and reimplantation were successful in all studied cases. Procedure time varied from five minutes to seven minutes for initial implantations. Repositioning took five minutes and retrieval took two minutes.
FLUOROSCOPIC EVALUATION
The implanted valves were closely investigated using fluoroscopy. Central prosthetic regurgitation was not present in any of the three animals. Paravalvular leak was present in two of the three cases: in one animal the paravalvular leak was mild by fluoroscopy, and in the other case paravalvular leak was mild to moderate. Three-dimensional evaluation of the stent was also undertaken using fluoroscopy, and the stents were found to be well apposed with no evidence of stent disruption or fracture in any of the cases. Two-dimensional measurements showed the valves were properly sized. Finally, aortic root angiography revealed patent coronary arteries in all three cases (Figure 5).
TTE EVALUATION
In each studied case, echocardiographic evaluation of FOLDAVALVE indicated proper valve function and alignment with no evidence of perforation or pericardial effusion in both 2D and 3D. Pulse wave Doppler of the valves yielded no evidence of stenosis with the maximum instantaneous velocity of 102 cm/s and a mean velocity of 70.2 cm/s in one case which corresponded to a maximum gradient of 4 mmHg and a mean gradient of 2 mmHg across the valve. Paravalvular leak was again confirmed in two cases, with one case being mild and one being mild to moderate according to colour Doppler studies (Online Figure 1). There was no evidence of central aortic regurgitation in any of the cases. Dobutamine was infused and a modified apical five-chamber view demonstrated no valve migration during or after infusion (Online Figure 2).
CLINICAL AND ANATOMICAL ASSESSMENT
The animals were clinically and haemodynamically stable throughout the procedure and through follow-up. None of the animals demonstrated evidence of heart block, significant bradycardia, atrial or ventricular arrhythmias on telemetry during or after implantation. None of the valves migrated or embolised during implantation or post-implantation monitoring. Macroscopic evaluation of the explanted hearts demonstrated that the valves were secure and in a stable position (Figure 6). There was no evidence of traumatic injury to the aortic root, left ventricular outflow tract or the thoracic aorta.
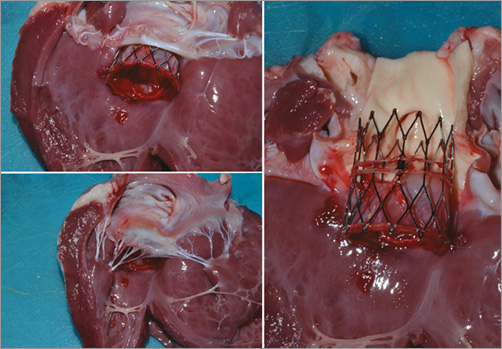
Figure 6. Images of the explanted ovine hearts after implantation of FOLDAVALVE. The valve has been naturally positioned within the native non-calcified aortic valve.
Discussion
Here we describe the proof of concept study of FOLDAVALVE, a novel self-expanding 25 mm transcatheter aortic valve with a 14 Fr delivery system. FOLDAVALVE is fully repositionable and retrievable, and spares the leaflets from the damage induced by crimping by means of excluding them from the stent during the crimping process (Figure 2, Moving image 1). The INGENUITY delivery system provides exceptional flexibility and control in addition to full repositioning and retrieval capabilities. Procedural success was also shown with successful animal implantations, no short-term complications, and multimodality imaging showing good valve function.
FOLDAVALVE addresses many of the challenges that will be faced in the quest to improve upon the currently available TAVs. In this short-term animal study, there were no instances of valve embolisation, coronary obstruction or heart block. The mild to moderate degree of paravalvular leak present in one case is believed to be due to the animal prosthesis size mismatch (Online Figure 1).
Through the use of a short-term animal model it was demonstrated that the valve and its delivery system were highly functional and provided the operator with meticulous control. By using a streamlined delivery system that excludes the leaflets from stent crimping and capitalising on a design with a physiologic flow profile13, FOLDAVALVE holds the promise of being a durable valve with excellent haemodynamics and, due to its low profile, a reduced rate of procedure-associated bleeding.
| Impact on daily practice TAVI is rapidly becoming a treatment option for more and more patients with severe AS. However, many TAVI delivery systems are bulky and induce a great deal of vascular trauma. The work presented here describes a TAV with advanced features such as repositioning and retrievability while maintaining a low-profile delivery system (14 Fr) and sparing the leaflets from crimping inside the stent. We have shown that implantation of this valve is feasible and that the functionality of the valve is good in a short-term animal model. |
Acknowledgements
The authors would like to thank Dr Nicolas Borenstein and his team from IMMR who assisted with animal studies. The authors would also like to extend their appreciation to Dr Jimmy Su, Dr Ahmad Falahatpisheh, Dr S. Hamed Alavi, Gregory Kelley, Edward Pannek and Pham Lo who helped with research and development of the valve and its delivery system.
Funding
This work was supported by a Coulter Translational Research Award (CTRA) from the Wallace H. Coulter Foundation.
Conflict of interest statement
A. Kheradvar is a co-founder and is associated financially and scientifically with FOLDA LLC, the parent company of FOLDAVALVE. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Schematic animation showing how leaflets are turning inside the stent via a drawstring mechanism to form a trileaflet heart valve.
Moving image 2. The six degrees of freedom in positioning when the valve is still attached to the delivery system.
Moving image 3. Transfemoral delivery, staged deployment, repositioning and complete implantation of FOLDAVALVE in sheep under fluoroscopy.
Moving image 4. Transfemoral delivery, staged deployment, implantation, retrieval and fresh implantation of FOLDAVALVE in sheep under fluoroscopy.
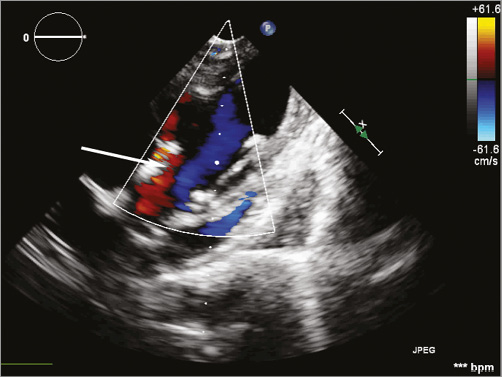
Online Figure 1. Colour Doppler echocardiogram of the valve in long axis with the paravalvular leak highlighted by the arrow. The leak is probably due to animal prosthesis size mismatch.
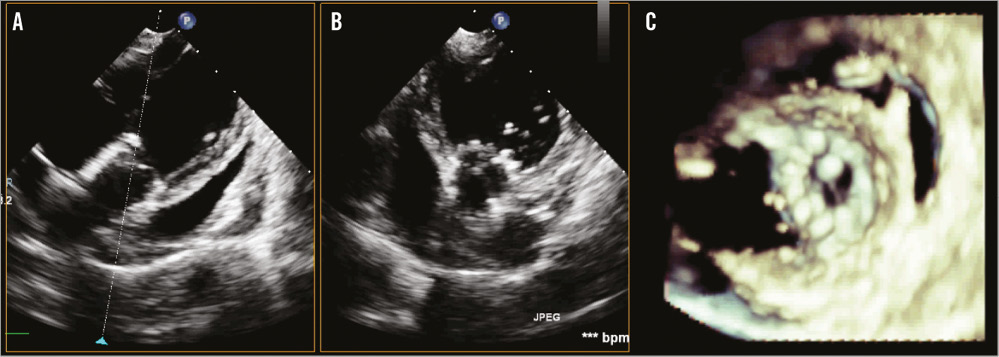
Online Figure 2. Two- and three-dimensional echocardiograms of the implanted FOLDAVALVE. A) & B) 2D echocardiograms of FOLDAVALVE in the long and short axis, respectively. The stent is well positioned and symmetrically expanded. C) 3D echocardiogram of the valve in short axis.

