
Abstract
Aims: Cardiac surgery in middle-income countries differs significantly from that in high-income countries regarding prevailing heart valve pathologies and access to cardiac surgery. Typically, rheumatic aortic regurgitation in the absence of calcification by far outweighs stenosis. As such, entirely different transcatheter aortic valve implantation (TAVI) concepts are required for these regions. The aim of the study was to evaluate the five-month performance of the SAT (Strait Access Technologies, Cape Town, South Africa) pericardial TAVI system in the orthotopic aortic position of juvenile sheep.
Methods and results: A self-homing, non-occlusive balloon-expandable TAVI system comprising a hollow balloon, stabilising locator trunks, a scalloped CoCr stent with elevating anchorage arms and decellularised, sandwich-crosslinked pericardium was compared with control surgical valves (Edwards PERIMOUNT) in sheep. The implantation period was five months. The tactile placement of the TAVI valves was accomplished without the need for rapid pacing. At termination, no structural degeneration was observed in either group. The TAVIs were well healed with the stent struts largely embedded in tissue. Correlating with sheep growth (weight gain of 40.4±13.0%) during the implantation period, mean transvalvular gradients increased from 3.08±1.95 mmHg to 8.50±5.38 mmHg (p=0.044) after five months.
Conclusions: A single-stage, balloon-expandable, easy to place TAVI system with antigen-depleted and antigen-masked bioprosthetic leaflets promises to address the distinct needs of low- and middle-income countries with regard to TAVI better than conventional systems.
Introduction
Transcatheter aortic valves were conceived for calcific aortic stenosis (AS) which is prevalent in the ageing population of North America and Europe1,2. Even in the few patients with predominant aortic regurgitation (AR)1, the underlying pathology in these regions is largely degenerative in nature1, often showing some degree of calcification. Contemporary valves used for transcatheter aortic valve implantation (TAVI) could therefore rely on the crushed mineral deposits for anchorage, obviating the need for stent features that could independently secure the TAVI valves in non-calcified, compliant roots. Therefore, with a few exceptions3,4, stent designs have continued to be based on smooth diamond-shaped elements. Naturally, when applied to non-calcific AR they had to be excessively oversized to avoid valve embolisation5, making it a suboptimal treatment choice. However, with four to five times more patients in high-income countries (HICs) suffering from calcific AS than AR1,2, this limitation of conventional transcatheter valves has still to become a clinical urgency in those countries.
In contrast, in middle-income countries (MICs), four times more patients are affected by non-calcific AR than calcific AS6 due to the prevalence of rheumatic heart disease (RHD)7,8,9. It is estimated that 56% of all patients requiring single aortic valve replacement (AVR) in these countries need it by reason of rheumatic AR7. As this percentage represents a mean value between a rapidly Westernising urban population and a rural majority7, a large proportion of patients requiring an AVR outside the reach of metropolitan centres need it for RHD. These patients are usually young. While even the non-rheumatic AR patients of HICs are on average 11-12 years younger than AS patients1, rheumatic AR patients in MICs are in their mid-forties when they come to surgery8,9. It is this large group of patients that needs a lateral solution outside the algorithms of HICs. First, only a fraction of these patients have access to cardiac surgery, due to limited capacity7. The majority of Chinese heart centres, for instance, operate on less than 100 cases per year7. Even in big urban centres, costs, availability and patient suitability limit the access to conventional transcatheter valve replacement. In 2016, only 160 TAVI procedures were performed in India and 900 in China7 (pro-rata corresponding German numbers would have been approximately a quarter of a million10). Secondly, the high failure rate of mechanical prostheses due to poor anticoagulation compliance9 and the early degeneration of bioprosthetic heart valves due to the young age of the patients adds urgency to the need for alternative long-lasting soft leaflet valves11.
With an estimated annual need for 360,000 single AVRs in Brazil, Russia, India, China, South Africa (BRICS) alone, where only 118,000 are currently provided, together with the relative paucity and low capacity of cardiac surgical centres7 and the young age of patients, an easy-to-place, long-lasting TAVI valve that is tailor-made for the population-specific pathology and addresses leaflet longevity would have the potential to expand capacity and improve the performance of valve prostheses in these regions.
In the current study, a TAVI system that has been developed to address the key challenges of MICs was evaluated in a chronic sheep model. These challenges include valve deployment in the absence of sophisticated imaging technology, the insertion into the hyperdynamic hearts of AR patients in the absence of anchoring leaflet mineralisation, and the use of a bioprosthetic material that significantly mitigates the accelerated leaflet degeneration anticipated in younger patients.
Methods
The aim of the study was to evaluate the five-month performance of the SAT (Strait Access Technologies, Cape Town, South Africa) pericardial TAVI system12 in the orthotopic aortic position of juvenile sheep. Implantation success in the compliant non-reinforced roots, healing, haemodynamic performance, calcification and valve integrity (structural and non-structural) were assessed.
TAVIS AND CONTROLS
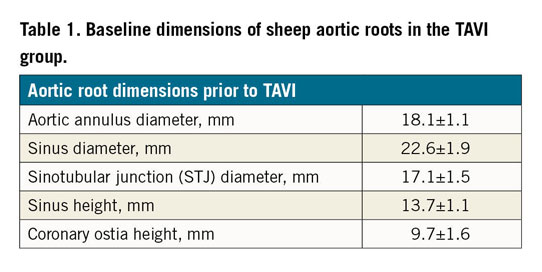
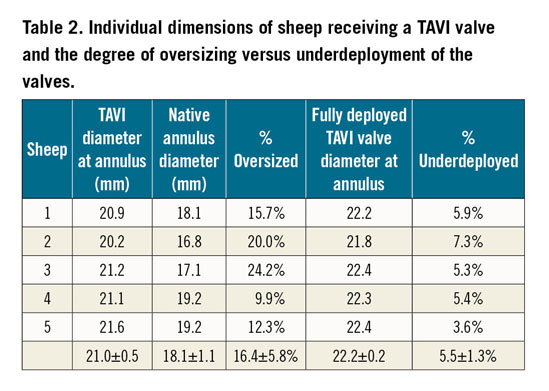
The self-locating, non-occlusive transapical deployment device comprises a unidirectionally flow-permissive hollow balloon with retractable location and stabilisation trunks and an invaginating retrieval sheath12 (Figure 1). The SAT-TAVI stent is based on a scallop design with expansion-linked arm protrusion for supra-annular anchorage (Figure 2). At the defined balloon filling pressure of 18 bar, 23 mm stents (n=10) are deployed to an annular diameter of 22.2±0.3 mm, with a distal diameter of 23.5±0.3 mm and a proximal diameter of 24.6±0.3 mm. The top arms are elevated to a diameter of 25.4±0.5 mm and the bottom supra-annular arms to 27.0±0.4 mm. Sub-scallop leakage is prevented by an electro-spun elastomer skirt welded to the stent. The decellularised13, sandwich-crosslinked14,15 bovine pericardial leaflets are continuously attached to the scallops. As controls, PERIMOUNT valves (size 19) (Edwards Lifesciences, Irvine, CA, USA) were implanted.
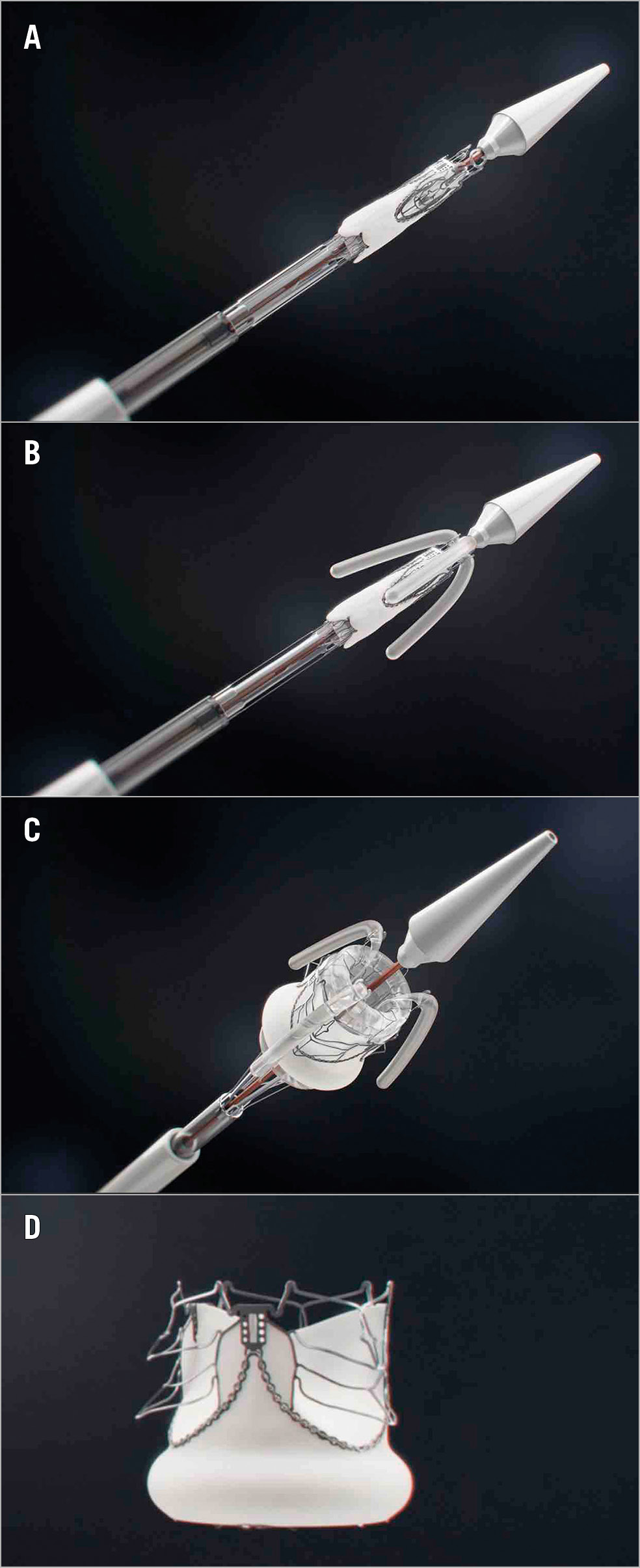
Figure 1. Key stages of the deployment of the self-homing, non-occlusive SAT-TAVI valve. Crimped SAT-TAVI system pushed out of the deployment sheath (A), with the locator and stabiliser trunks deployed (B) followed by the full expansion of the scalloped, self-anchoring stent (C). The cobalt-chromium stent is designed to lift up six arms through plastic deformation (D). All arms are seated supra-annularly creating sinus-like outward bulges of the leaflets that firmly anchor the stent in the absence of leaflet calcification.
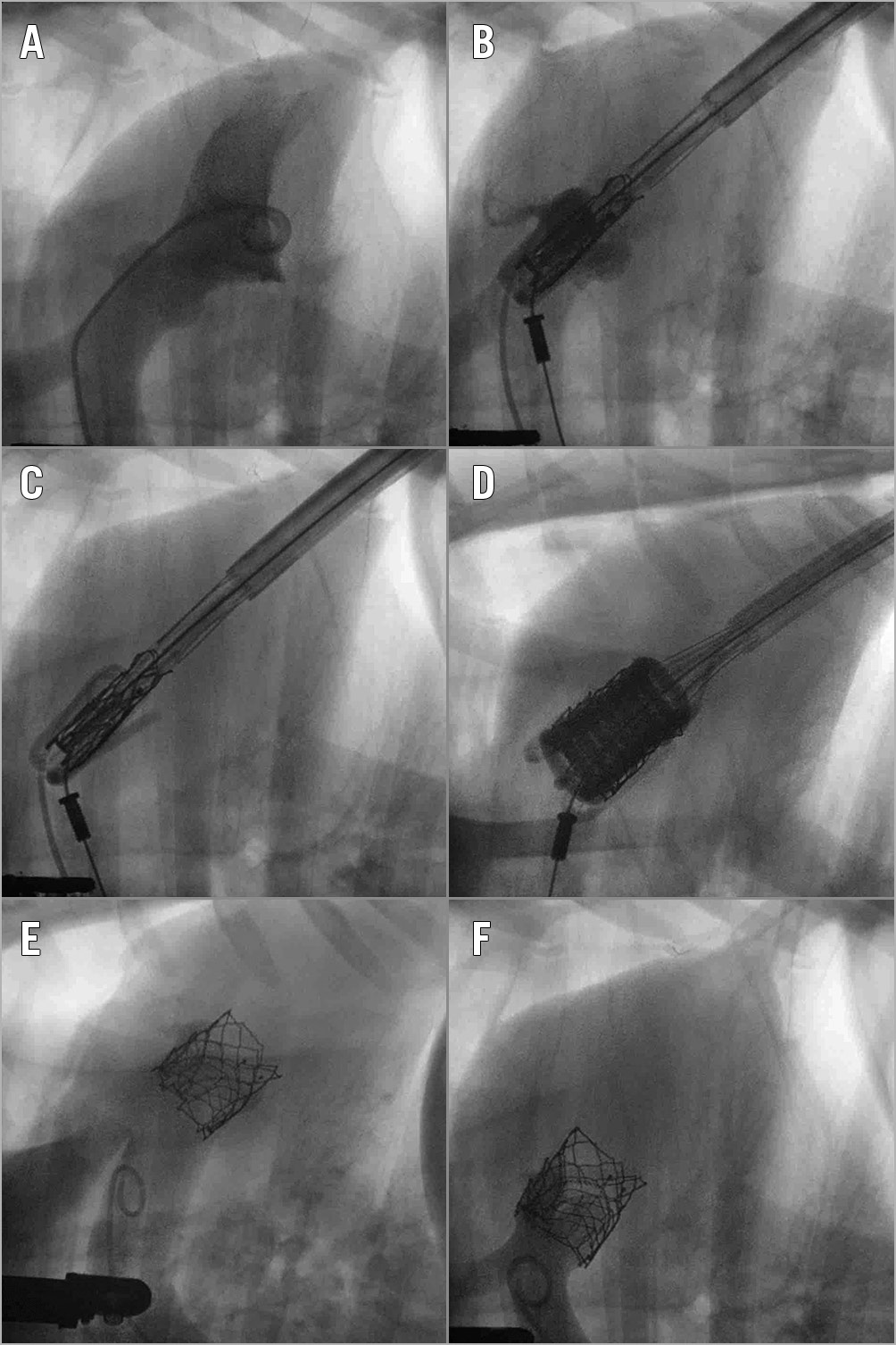
Figure 2. Fluoroscopy-guided transapical insertion of the SAT-TAVI valve. Following the visualisation of the aortic root through a contrast medium injection (A & B), the self-locating balloon trunks are inflated (C) followed by gentle traction on the system providing tactile feedback for the trunks engaging in the native aortic valve cusps, thereby ensuring optimal positioning and root stabilisation. The valve is deployed by inflating the hollow balloon (D), without rapid pacing. Following deployment, coronary perfusion and absence of regurgitation are angiographically confirmed (E & F).
SURGICAL PROCEDURES/TAVI DEPLOYMENT AND POSTOPERATIVE FOLLOW-UP
The study was approved by the Faculty of Health Sciences Animal Ethics Committee, University of Cape Town (AEC 016/015). To compensate for the oversizing inherent to TAVI, larger animals were used for the surgical control group (53.8±2.8 kg/12 months of age) than for the TAVI group (37.8±2.4 kg/10 months old). Preoperatively, animals were screened with transthoracic echocardiography (Vivid I BT09; General Electric, Horten, Norway) to pre-assess aortic dimensions. Group 1 (n=5) underwent a transapical insertion of a SAT pericardial TAVI valve (size 23 mm) in the orthotopic position16 and Group 2 (n=5) underwent a surgical AVR (sAVR). In brief, the SAT-TAVI deployment system is transapically inserted and advanced into the ascending aorta under fluoroscopic and echo control allowing the confirmation of root dimensions (Table 1, Table 2). Following the deployment and engagement of the downward-pointing balloon trunks into the nadirs of the leaflets and confirmation of rotational alignment and position on fluoroscopy, the tactile feedback allows the operator to apply a continuous gentle pull to stabilise the moving valve plane while guaranteeing the correct position for the TAVI placement. Rapid inflation of the hollow deployment balloon allows full expansion of the TAVI valve stent and the six supra-annular anchoring arms while the temporary back-flow valve in the hollow balloon maintains normal diastolic pressures for coronary perfusion. After deflation of the stabilising balloon trunks and the deployment balloon, both are engulfed by a pressurised rolling-sheath for atraumatic retrieval.
Low-dose antiplatelet therapy (aspirin 50 mg daily) was commenced on day 1; clinical evaluations were performed daily. After the animals were returned to pasture, the antiplatelet regimen was continued until termination. At one and three months postoperatively, transthoracic echocardiograms (TTE) were performed.
EXPLANT PROTOCOL
Termination (sodium pentobarbitone 200 mg/kg iv and K-chloride 3 g) was performed five months (152±3 days) postoperatively, after prosthesis function had been evaluated by echocardiography and fluoroscopy. Valves were explanted for macrophotography and histological assessment (haematoxylin/eosin, Brown-Brenn, Von Kossa stains). Calcium content was determined by lyophilisation and ashing, dissolution in hydrochloric acid, with measurement by inductively coupled plasma-atomic emission spectroscopy (ICP-AES), the results expressed in μg/mg of dry weight.
STATISTICAL ANALYSIS
Inferential statistical analysis was performed using the JMP statistical software package, version 13.0.0 (SAS Institute Inc., Cary, NC, USA). Distribution of continuous numerical data was evaluated using the Shapiro-Wilk test. Categorical variables were presented as frequencies (%) and continuous variables were reported as means±standard deviation. Parametric continuous data were analysed using the Student’s t-test and non-parametric data using the Wilcoxon test.
Results
All ten consecutive sheep underwent successful AVR. Except for one animal in the control group that died of valve infection on day 120, all reached the five-month observation endpoint. Two transcatheter valves that had been placed too low during deployment due to an entrapped control line of a locator arm had their resulting mild to moderate paravalvular leak (PVL) continually detectable at months 1 and 3 and at termination.
At implantation, surgical control valves had been largely size-matched with the native annulus of the sheep while TAVI valves were diameter-oversized by 16.4±5.8% and underdeployed by 5.4±2.3% diameter (range: 2.7-9.0%). The tactile placement of all TAVI valves was accomplished within 9.6±2.2 minutes after transapical entry without the need for rapid pacing. Systolic pressures dropped only mildly by 17±2% during transapical entry (from 103±13 mmHg to 85±12 mmHg) and 26±11% during actual deployment (from 100±20 mmHg to 72±9 mmHg). Correspondingly, diastolic pressures were maintained at a mean of 65.4±9.1 mmHg during the inflation of the deployment balloon, confirming the echo finding of an effective temporary back-flow valve inside the hollow balloon.
In the TAVI group, which experienced a weight gain of 40.4±13.0%, after five months, mean transvalvular gradients increased from 3.08±1.95 mmHg to 8.50±5.38 mmHg (p=0.044; Student’s t-test) and maximum transvalvular gradients from 8.04±5.13 mmHg to 20.90±9.36 mmHg (p=0.035). In the older control group, which experienced only a 14.3±5.3% weight increase, after five months, mean transvalvular gradients decreased from 14.58±2.64 mmHg to 10.53±2.47 mmHg (p=0.066) and maximum transvalvular gradients from 35.23±5.39 mmHg to 19.55±7.13 mmHg (p=0.014).
Apart from the animal implants, separate pulse duplicator tests (ISO 5840-5L/min) were also performed on 10 SAT-TAVI and two surgical control valves which showed effective orifice areas (EOA) of 1.95±0.07 cm2 and 1.46±0.25 cm2, a transvalvular closure leakage of 0.83±0.90% and 1.56±1.02%, and a total closing plus post-closure regurgitation volume of 4.38±1.65% and 1.79±0.08%, respectively.
MACROSCOPIC APPEARANCE
No structural degeneration was observed in any explanted valve (Figure 3A-Figure 3J). All leaflets were free of blood clots. On the aortic side, stent struts of TAVI valves were largely embedded in tissue and white glistening neointimas covered most of the leaflets (Figure 3A-Figure 3D). All coronary ostia were widely patent. Native leaflets had largely shrunk, resulting in a singular sinus space between the TAVI leaflets and the aortic wall (Figure 4). Control valves showed distinct, white tissue overgrowth onto the cloth-covered stent posts but otherwise did not differ significantly from the TAVI group. On the ventricular side, both groups showed a significant pannus shelf formation consisting of a whitish, aperture-like, sharp-edged tissue and wart-like, flat microthrombi in the dead space underneath the commissures and on the leaflets (Figure 5).
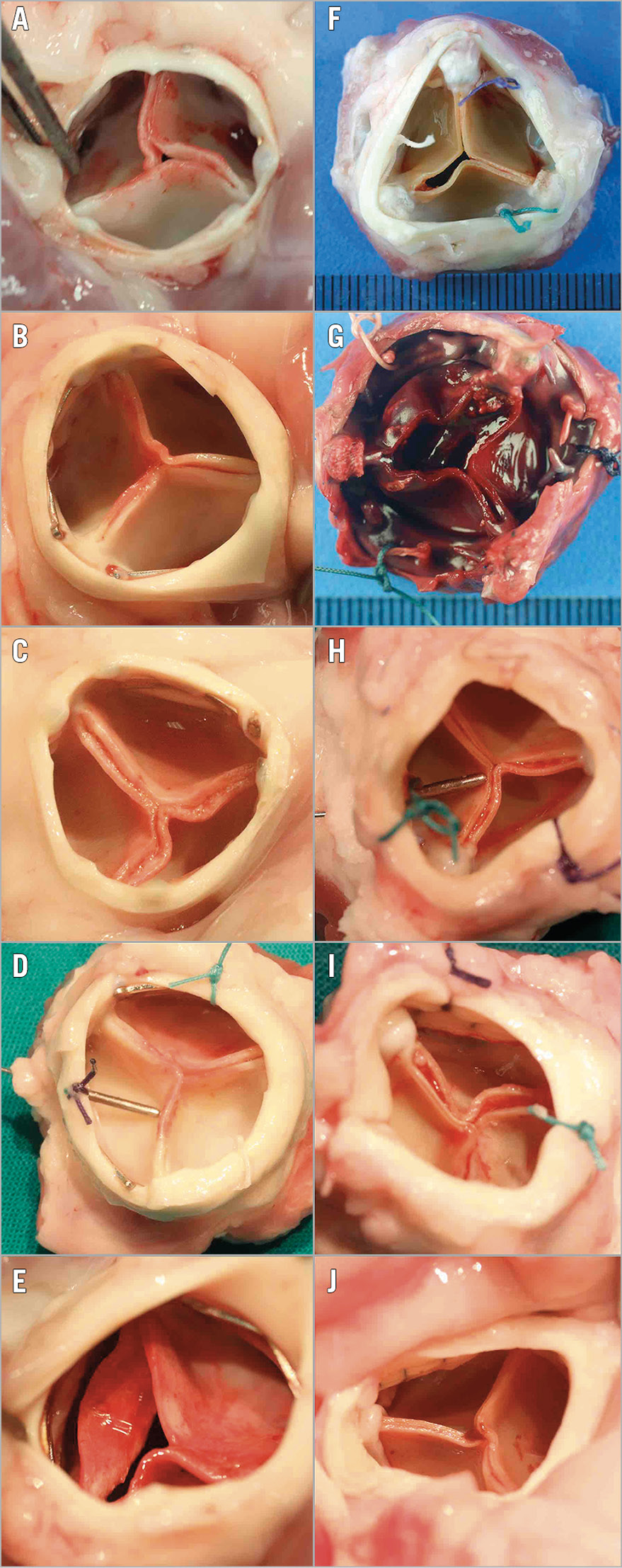
Figure 3. Five-month implants. Explant macro-photographs of SAT’s pericardial TAVI (left column A-E) and Edwards PERIMOUNT control valves (right column F-J) after five months in orthotopic position in sheep. Only one sheep died prematurely on day 120 (G) with the vegetations of the prosthetic endocarditis visible between the commissures (post-mortem picture). The whitish neointimal outgrowth is visible on the aortic side of the leaflets in both groups but more complete in the TAVI group where it reached the cusp edges in 4/5 valves (A-D). The TAVI stents are well embedded in tissue with only the distal commissural tips being visible. The four long-term control valves show distinct pannus outgrowth onto the fabric-covered stent posts.
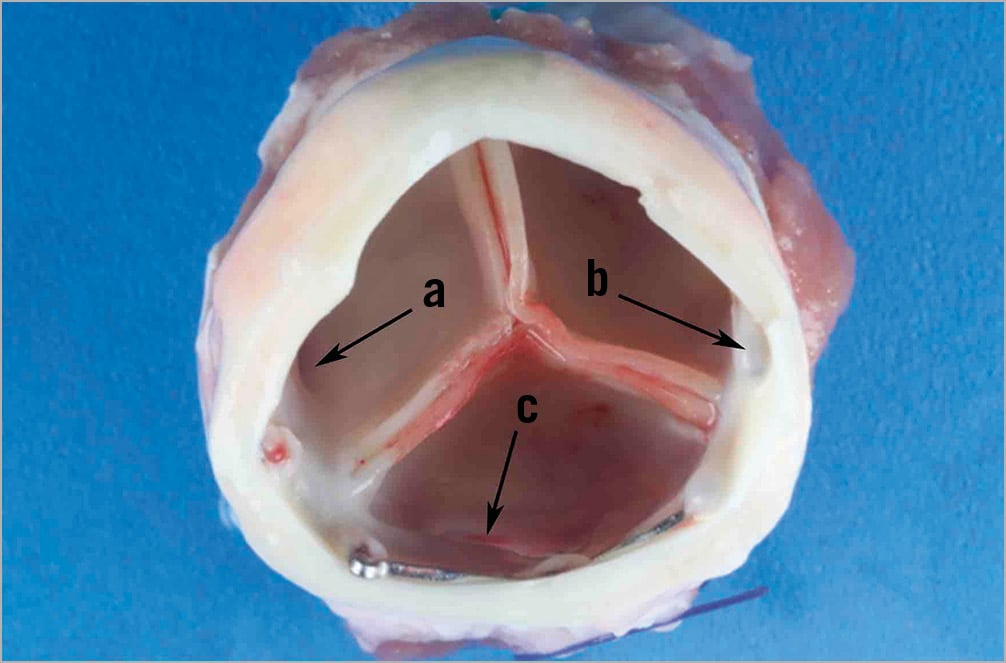
Figure 4. Outflow/aortic view. Remodelled sinus region after SAT-TAVI placement with both the left (a) and the right (b) coronary ostium visible. Both native leaflets and the edge of the skirt have been integrated into the “neo-sinuses” without compromising the ostia of the coronaries. The edge of the stretched native non-coronary leaflet is still visible (c).
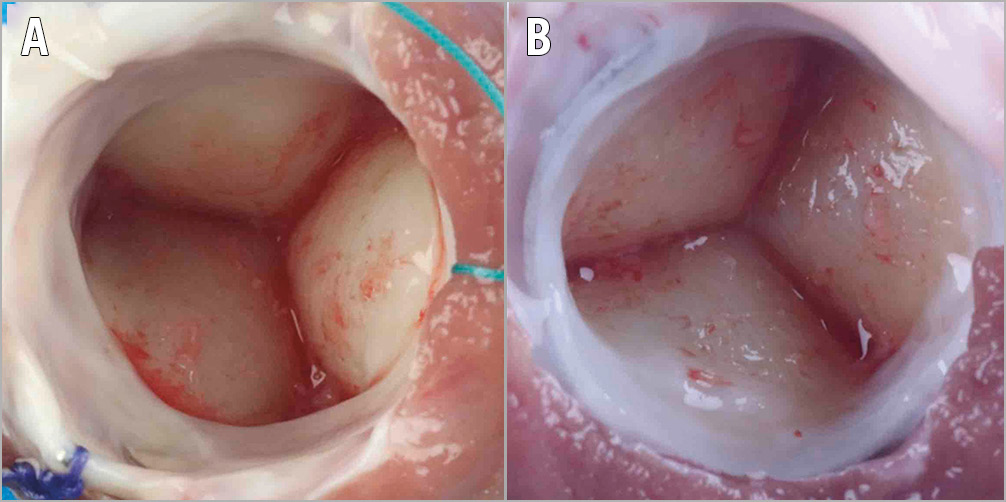
Figure 5. Inflow/ventricular view. View of a TAVI valve (A) and a control valve (B) from the ventricular side. In both groups, typical infravalvular pannus shelves formed and neointimal outgrowth was scarce.
HISTOLOGY
Upon explant, leaflets were 41% thinner in the control group (438±52 μm) than in the TAVI group (737±77 μm). Both groups showed tapering pannus wedges at the base of the leaflets (Figure 6). On the aortic side, they continued as neointima of varying thickness covering most of the leaflet surface. This was more pronounced in the TAVI group where the neointima reached the cusp edge, forming a round cap (Figure 6A). On the ventricular side, neointimal coverage was sparse. The macroscopically visible small microthrombi consisted of pure platelet aggregates (Figure 6A, Figure 6B). The electro-spun skirt of the TAVI group showed complete transmural vascular ingrowth in the areas of tissue contact at the commissures and the annulus (Figure 6C, Figure 6D), while in areas without tissue contact cell infiltration was patchy and lacking blood vessels.
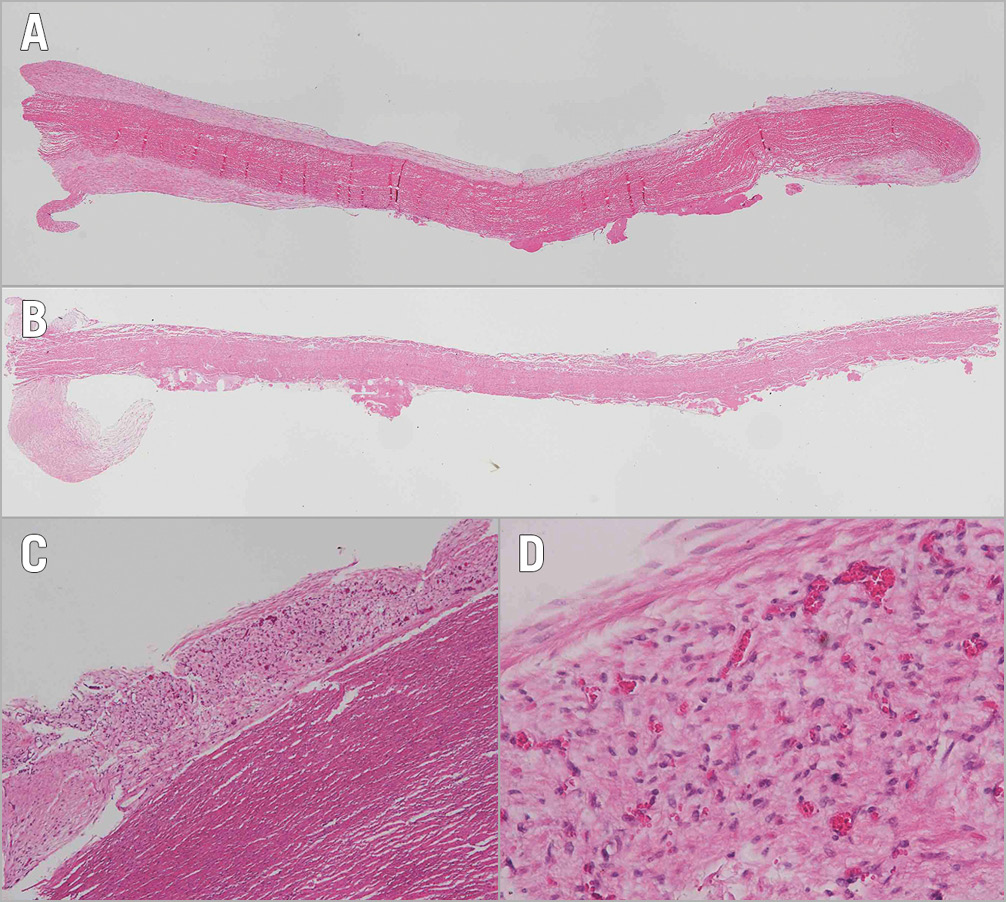
Figure 6. Longitudinal cross-sections of leaflets. The decellularised, sandwich-fixed pericardium of the TAVI valve (A) is thicker than the PERIMOUNT leaflet (B) with clearly visible neointimal outgrowth onto the aortic surface. The infravalvular pannus shelves are visible on both groups. The TAVI valve skirt shows complete transmural tissue ingrowth in the proximity to the aorta (C) with numerous blood vessels throughout (D).
CALCIUM ANALYSIS
Histologically, no traces of calcification were observed in either group. ICP-AES analysis confirmed similarly low calcium values for TAVI versus control groups in individual leaflets (8.4±19.6 versus 17.0±36.8 µg/mg; p=0.437) and when combined (8.4±16.0 versus 17.0±27.2 µg/mg; p=0.782) (Wilcoxon test).
Discussion
Apart from cost-effectiveness, TAVIs need to address very different requirements in middle- and high-income countries. The specific requirements in MICs include independence from sophisticated imaging equipment, the ability to locate and anchor the valve in the absence of calcification, the acknowledgement of resource limitations that necessitate single-stage procedures and are near prohibitive for potential downstream interventions such as permanent pacemakers (PPMs), the appreciation of the predominance of rheumatic aortic regurgitation with its associated fundamentally different haemodynamics, and the need for long-lasting leaflet materials for use in younger patients.
We have previously shown12 that these specific requirements can be addressed by a purpose-designed transapical balloon-expandable TAVI system. The current chronic study confirmed the ability of the delivery system to deploy a balloon-expandable TAVI valve without the need for rapid pacing and with ejection maintained throughout cardiac systole. It also showed that tactile placement allows accurate positioning. The entrapment of the control line of the balloon trunks that led to too low a deployment in two sheep has since been addressed.
As the positioning trunks consist of smooth yet bend-resistant 4 mm balloons, they do not have the traumatising potential of metal arms which may cause dissections17. The trunk retrieval through invagination at the end of the procedure guarantees a friction-free withdrawal, preventing dislodgement of the deployed valve. An extremely tight aortic root model such as the sheep also demonstrated the ability of the balloon trunks to maintain sufficient inflow spaces to the coronary ostia when the helical deployment balloon was fully inflated. Despite tightly stretching the sinotubular junction, the fully inflated balloons did not cause any signs of ischaemia. Confirming ex vivo tests, this additional safeguard of coronary perfusion warrants sustained maximal balloon inflation at the conclusion of the deployment, thereby potentially minimising paravalvular leaks and ellipticity. The hollow balloon itself providing a luminal cross-sectional area of 1.8 cm2 for a 23 mm valve and an effective backflow valve that maintains sufficient diastolic pressures for coronary perfusion was haemodynamically almost “invisible”.
The supra-annular stent arms resting on the ventricular side of the leaflet nadirs proved to be firm and effective anchors in the compliant non-calcified sheep roots. Once deployed, none of the valves migrated, dislodged or embolised. More than in pigs or calves, the over-elastic annulus of sheep had previously been shown to lead to device migration and paravalvular leaks unless stiffening reinforcements were pre-implanted. Our elevating stent arms added an essential feature to balloon-expandable TAVI valves that has, until now, only been achievable with self-expanding valves. Purely on the basis of expansion-deformation of the cobalt-chromium stent, the radius of the fully deployed arms exceeded the annular stent diameter by 25%.
The scalloped attachment struts for the leaflets were designed to deploy to their perfect pre-crimping shape. This was confirmed on implantation fluoroscopy. We had previously shown that this scallop design also allows the continuous attachment of durable polymeric leaflets12. Particularly for the latter, optimisation of leaflet strain through avoidance of excessive oversizing made a balloon-expandable concept additionally preferable. The de facto underdeployment of valves in the present sheep study was at a modest 5%, preserving near-perfect leaflet geometry.
Although current guidelines for TAVI in middle-income countries mirror those of high-income countries, the slow but continual global trend towards younger patients has a different long-term connotation in countries such as Brazil, South Africa, India or even China7 compared to Europe or the USA. In MICs, surgical valve replacements are far from representing a “gold standard”, as the mechanical prostheses prescribed for the largely young, rheumatic patient populations perform suboptimally under the prevailing circumstances9. Yet, adherence to the guidelines of high-income countries has so far prevented decisive head-to-head studies between modern tissue valves and mechanical valves. However, should such studies eventually show an overall benefit of soft leaflet valves in the affected populations, a major hurdle towards using simple transcatheter therapies to augment the insufficient capacity of open heart surgery in these countries would have been overcome7. The implementation of scientific advances in bioprosthetic tissue preservation, therefore, has a higher priority for middle- than for high-income countries with their predominance of older patients combining limited life expectancy with slower bioprosthetic tissue degeneration. In this regard, the recent two-pronged approach towards eliminating remnant immunogenicity18 holds great promise. One arm of this approach comprises the removal of cells as they represent the bulk of antigen carriers19. Various decellularisation approaches have since successfully found their way into clinical implementation20. Concomitantly, a higher efficacy of antigen masking through improved sandwich-crosslinking was also shown to mitigate calcification significantly14. By combining both approaches, pericardial calcification could be abolished (from 127 µg/mg to 3 µg/mg; p<0.001) in the commonly accepted rat model (unpublished data).
For size reasons, the current study used 10- to 12-month-old sheep. As the sheep ceases to be a calcification model once older than four months, the present study was not expected to provide validation of the tissue preservation process with regard to mineralisation. Yet, the pristine histomorphology of all explants, and the complete absence of inflammatory cells at both the surface of and inside the decellularised pericardium suggested the absence of a significant immune response of the TAVI pericardium. With the choice of PERIMOUNT surgical valves as controls, a latest-generation tissue valve was selected. Other than the thinner pericardium used in these control valves, the two groups barely differed in their explant macro-morphology, neither in their aperture-like subvalvular tissue shelf nor in the platelet microthrombi on the ventricular side. Neointimal outgrowth was more pronounced on the TAVI valves covering the entire aortic side of the leaflets. This may have been due to the healing ability of the electro-spun skirt that facilitated complete transmural vascularisation in areas of direct tissue contact with the aorta or myocardium. We believe that the unprecedented transmural endothelialisation through the skirt may also hold the key to future tissue regenerating TAVI concepts, as transmural ingrowth was recently shown to be the only significant source of prosthetic surface endothelialisation21 other than transanastomotic pannus outgrowth22.
Limitations
The present small series successfully demonstrated the feasibility of implanting the SAT pericardial TAVI system in the orthotopic aortic position of juvenile sheep. Although the five-month follow-up data presented herein are very encouraging, follow-up studies may be required to confirm these findings in a larger preclinical series.
Conclusions
The present study showed the promising five-month performance of the SAT pericardial TAVI valve in sheep, validating the key characteristics underlying a transcatheter system that was specifically designed to address current and future requirements in middle- and potentially even low-income countries. By providing a self-homing and stabilising balloon-expandable TAVI system that obviates fast pacing, several prevailing paradigms were disproved:
– Self-homing locator arms do not need to be part of self-expanding stents but can be balloon-based and be part of the dilatation balloon. By retraction through invagination, they were proven to resist pinching even under the extremely tight conditions of the sheep model.
– Protruding stent structures required for the anchorage of TAVI valves in compliant non-calcified annuli do not need to rely on the shape-memory feature of self-expanding stents or two-component designs. By plastic deformation of a cobalt-chromium stent during balloon inflation, firm anchorage was achieved in the hyperelastic sheep model without excessive oversizing.
– Rapid pacing does not need to be an integral part of balloon-expandable TAVI valves. By utilising a widely open helical balloon, the TAVI valve deployment was possible at the required radial forces, with continuing unimpeded ejection.
– The presence of a “shielding” cloth skirt does not preclude tissue ingrowth from the surrounding tissue. The electro-spun skirt allowed full transmural vessel ingrowth and may have facilitated the complete neointimal coverage of the adjacent leaflets.
– The integration of scallops into cobalt-chromium stents, and the direct attachment of the leaflets to these scallops do not result in an uneven post-crimping shape and detrimental stress concentrations. The structural integrity of the leaflets after five months in the sheep confirmed fatigue test data of >800 million cycles.
The successful realisation of a stent design that allows direct attachment of leaflets to scallops also allowed us to pursue a twin version with polymeric leaflets. After proof of concept and acute animal implants12, a chronic sheep study has commenced with the SAT polymeric TAVI system.
|
Impact on daily practice The present study validated in the chronic sheep model a transcatheter system that was specifically designed for the largely young patients in need of aortic valve replacement for rheumatic heart disease with predominant aortic regurgitation. Having overcome the disadvantages of conventional balloon-expandable TAVIs, a single-stage balloon-based procedure can be offered that takes both the specific pathology and the resource-constrained circumstances of low- to middle-income countries into account. |
Acknowledgements
The authors wish to thank Dr Richard Bianco for his invaluable advice regarding the surgery of the control group.
Funding
The present study was funded by Strait Access Technologies.
Conflict of interest statement
D.F. Williams, D. Bezuidenhout and P. Zilla declare a potential conflict of interest, being Directors of Strait Access Technologies. B. van Breda and H. Appa are employees of Strait Access Technologies. J. Scherman and C. Ofoegbu consult for Strait Access Technologies. The other authors have no conflicts of interest to declare.

