Device description
Name and manufacturer: AccuFit™ transapical mitral valve replacement (TMVR) system; Sino Medical Sciences Technology Inc., Tianjin, China.
Approval status: Preclinical. Acute animal studies are now in the final stages. The EU first-in-man trial will be initiated in the third quarter of 2016.
Platform: Nitinol.
Specific design (Figure 1): The self-expanding, self-centring AccuFit device body has an atrial flange, a ventricular flange (maximum height 14 mm) and an annulus support. The annulus support includes a ring of anchors extending radially, with an annular clipping space between the atrial flange and the ring of anchors. A set of tails extends from the ventricular side for controlled deployment. The three bovine pericardial leaflets are secured to the leaflet holders positioned inside the atrial flange at a location above the native annular plane. The leaflet design is uniquely reversed with a central attachment and a circumferential coaptation (Moving image 1).
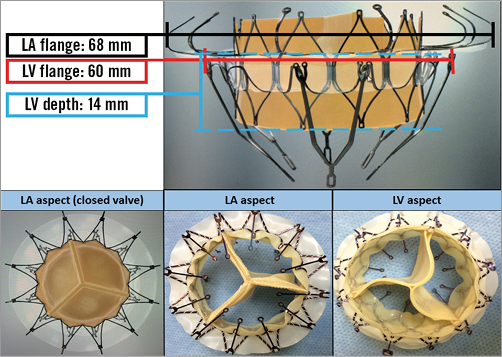
Figure 1. The AccuFit™ TMVR prosthesis. The atrial aspect (left, lower) and the ventricular aspect (right, lower) displaying the reverse leaflet design.
Delivery/approach system: The transapical delivery system calibre is 38 Fr and is compatible with a 0.035” or a 0.038” guidewire. The system is made of polyvinylidene fluoride (PVDF) and polytetrafluoroethylene (PTFE) to reduce system friction with a smooth nose cone to facilitate transition through the apex and the mitral valve complex.
Device sizes/lengths: The valve is currently available in one size (38 mm). Forthcoming valve sizes will be from 30 to 42 mm, in 4 mm increments. Sizing is based on echocardiographic mitral valve (MV) orifice average dimension ([mediolateral dimension+anteroposterior dimension]/2) with a manufacturer-recommended oversizing of 3 mm. The folded diameter of the 38 mm valve is 10 mm.
Procedural details (Moving image 2)
The valve is mounted on-site using a loading tool. Using a transapical approach, a guidewire is passed through the native MV into the left atrium and the delivery system with the mounted AccuFit TMVR device is then inserted over the guidewire and positioned within the MV under echocardiographic and/or fluoroscopic guidance. The atrial followed by the ventricular clips are unsheathed forming an hourglass. The waist of the hourglass is positioned to encompass the MV annulus and the subvalvular apparatus. At this point of deployment, the device can be resheathed and repositioned if necessary to achieve a more optimal placement. The ventricular legs are then advanced until they are deployed, and the fully expanded section can be used to entrap the leaflets. Thereafter, the atrial legs are released, completing the clipping mechanism and providing a firm anchor of the device to the MV annulus. Finally, the TMVR device is released from the delivery system which is then removed, and the apical access is closed with pre-made purse-string sutures.
Preclinical data
Phantom-heart implants revealed adequate coaptation under various valve frame contour configurations. No significant malcoaptation could be detected when the valve frame adopted an oval shape (Moving image 3) or a D-shape (Moving image 4), rather than a circular configuration.
Successful device delivery and positioning through a transapical procedure have been confirmed in an acute animal study. A pilot chronic animal study included nine LYD cross-breed Yorkshire swine (weight at intervention: 80-90 kg). Two pigs were serially monitored with echocardiography (at 30, 60 and 90 days) and electively sacrificed at 98 days post implantation. Throughout the follow-up period echocardiography revealed no evidence of left ventricular outflow tract (LVOT) obstruction or more-than-mild valvular or paravalvular mitral regurgitation (MR). The explanted hearts showed a fully healed left atrial flange with no thrombus formation and no evidence of frame fracture or leaflet malfunction.
The feasibility of the procedure has also been shown in a human cadaveric model. Two recently deceased heart/lung blocks were infused with non-pulsatile pressurised saline to simulate typical left ventricular and left atrial volumes. Uneventful deployment with visual seal of the mitral annulus could be achieved under epicardial echocardiographic and fluoroscopic guidance (Figure 2).
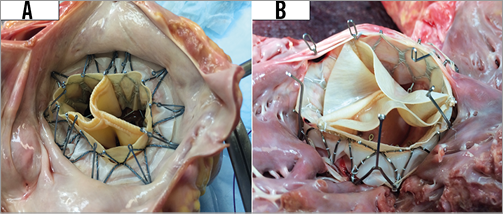
Figure 2. Deployment in a human cadaveric model. A) A left atrial view of a successful implant into a recently deceased heart block from an 83-year-old female who died of sepsis: ht: 5’0”, wt: 75 lbs. B) A left ventricular view of a successful implant into a recently deceased heart block from a 63-year-old male who died of laryngeal cancer: ht: 5’10”, wt: 195 lbs.
Ongoing studies
Ongoing chronic animal studies include 12 pigs with a scheduled sacrifice at 30 days (three animals), 60 days (three animals) and 90 days (six animals). The periprocedural endpoints include procedural success and periprocedural complications. The intermediate-term (till the time of scheduled sacrifice) endpoints include valve performance (by echocardiography and fluoroscopy), complications (clinical, mechanical, electrical and haematological), and adverse host cellular reactions to the device (explant gross and histopathologic examination). The results will be available later this year. The EU first-in-man trial will be initiated in the third quarter of 2016.
Unique features
The unique reverse leaflet design with a circumferential coaptation (Moving image 1) eliminates central leakage, even if annular remodelling leads to stent deformation (Moving image 2, Moving image 3). The anchor mechanism is achieved mainly through sandwiching the annulus and native valve leaflets (clipping effect) between the atrial and ventricular flanges using 12 nitinol clips, rather than through mere radial force. Connected to each side of the clip, a skirt of PET fabric helps perivalvular sealing and encourages tissue ingrowth with an overall objective of reducing the risk of paravalvular leakage. The design thus specifically targets elimination of transvalvular and paravalvular leakage (both are probably poorly tolerated due to the risk of haemolysis and poor healing from high velocity) without excess radial force for anchoring, thus keeping the left ventricular outflow unimpeded.
Potential improvements
Ongoing improvements include the delivery system (targeting a more convenient and stable handling during transapical delivery), valve platform (targeting less metallic components, smaller profile of the mounted valve, and thus the probability of transfemoral delivery) and prosthetic valve leaflets (targeting improved durability).
Conflict of interest statement
J. Ma is an employee of Sino Medical Sciences Technology Inc. The other authors have no conflicts of interest to disclose.
Online data supplement
Moving image 1. The unique leaflet design of the AccuFit TMVR prosthesis eliminates central leakage.
Moving image 2. The implantation procedure of the AccuFit TMVR prosthesis.
Moving image 3. Atrial view of the AccuFit valve displaying good coaptation despite an oval-shaped frame.
Moving image 4. Atrial view of the AccuFit valve displaying good coaptation despite a D-shaped frame.
Supplementary data
To read the full content of this article, please download the PDF.
Video 1. The unique leaflet design of the AccuFit TMVR prosthesis eliminates central leakage.
Video 2. The implantation procedure of the AccuFit TMVR prosthesis.
Video 3. Atrial view of AccuFit valve displaying good coaptation despite an oval-shaped frame.
Video 4. Atrial view of AccuFit valve displaying good coaptation despite a D-shaped frame.

