Device description
Name and manufacturer: Medtronic transcatheter mitral valve; Medtronic, Minneapolis, MN, USA.
Approval status: Preclinical.
Platform: Nitinol.
Specific design: Self-expanding nitinol frame consisting of an inflow (“atrial portion”) and outflow (“ventricular portion”) with support arms securing the position of the device (Figure 1).
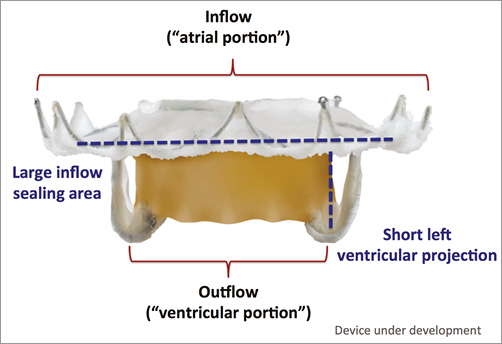
Figure 1. The Medtronic transcatheter mitral valve. The valve has a large inflow (“atrial portion”) to help seal and prevent paravalvular mitral regurgitation. The outflow (“ventricular portion”) is short and designed to avoid left ventricular outflow tract obstruction. Two diametric support arms function to capture the A2 and P2 segments of the mitral valve leaflets.
Delivery approach: Transatrial.
Device sizes/length: Not specified.
Procedural details
The current device is delivered via the transatrial approach using a right lateral minithoracotomy, similar to minimally invasive surgical mitral valve repair. The transatrial approach is intended to avoid trauma to the left ventricle, which is a possible risk associated with the transapical approach. The delivery system is inserted into the left atrium at Waterston’s groove and advanced antegradely across the mitral valve into the left ventricle. The two diametric support arms are oriented towards the anterior and posterior mitral valve leaflets. Under fluoroscopic and echocardiographic guidance, the support arms are exposed below the tips of the mitral valve leaflets. The delivery system is retracted slightly in order for the support arms to engage and capture the A2 and P2 segments of the mitral valve leaflets. After entrainment of the mitral valve leaflets, the outflow (“ventricular”) and inflow (“atrial”) portions of the valve are deployed sequentially. Valve position and function are evaluated by angiography and echocardiography. In cases of malpositioning, for example, the device can be fully recaptured and retrieved from the body prior to full release from the delivery catheter.
Clinical data
There are no clinical data as yet available.
Ongoing studies
Acute animal studies have established the safety and feasibility of the procedure. These studies have confirmed the device can maintain an accurate position during deployment and maintain a stable position after its release. Furthermore, there was absence of paravalvular regurgitation, left ventricular outflow tract obstruction and significant transvalvular gradients in the vast majority of animal experiments. Post-mortem examinations have ruled out injury to the mitral valve leaflets or their apparatus after recapturing the device. Chronic animal studies are ongoing.
Unique features
Evidently, bail-out options surrounding transcatheter mitral valve replacement are potentially more challenging than those associated with transcatheter aortic valve replacement. The Medtronic mitral transcatheter valve system is recapturable and retrievable after complete deployment and assessment of valve function. This may avoid the need for bail-out surgery in cases of significant paravalvular mitral regurgitation, left ventricular outflow tract obstruction or device malpositioning.
Potential improvements
A transseptal system is currently in development.
Conflict of interest statement
N. Piazza is a member of the Scientific Advisory Board of Medtronic and a consultant and equity shareholder with HighLife. H. Treede is a member of the Scientific Advisory Board and a Proctor with Medtronic and a consultant with HighLife. N. Moat is a member of the Scientific Advisory Board of Medtronic and a consultant with Tendyne. P. Sorajja is a member of the Scientific Advisory Board of Medtronic and a consultant with Abbott MitraClip. J. Popma, E. Grube, S. Bolling and D. Adams are all members of the Scientific Advisory Board of Medtronic.

