Device description
Name and manufacture: HighLife SAS, Paris, France.
Approval status: Preclinical.
Platform: Nitinol.
Specific design: The HighLife transcatheter mitral valve replacement device is a two-component system (Figure 1). The valve prosthesis is implanted over a delivery system in the mitral position and is anchored by interacting and then reaching an equilibrium position with a previously positioned subannular implant (SAI). The valve prosthesis shape has a preformed groove in the annular region so as to create an interference with the loosely placed SAI. The SAI is placed around the prosthesis groove, which blocks it from slipping into the atrium, whereas the inflow shape of the stent itself blocks it from slipping in the opposite direction into the ventricle. The combined dynamic of these two opposite interactions results in a stable position in the heart (which could be described as an equilibrium position that adapts to different anatomies). The HighLife transcatheter mitral valve (TMV) is a stented mitral bioprosthesis made from glutaraldehyde cross-linked bovine pericardium mounted on a laser-cut nitinol frame. The SAI is intended to create a closed loop with a fixed perimeter around the patient’s native valve leaflets and chordae. In its final position, it allows the TMV to remain in a stable position in the heart, the native leaflets being positioned between the SAI and TMV.
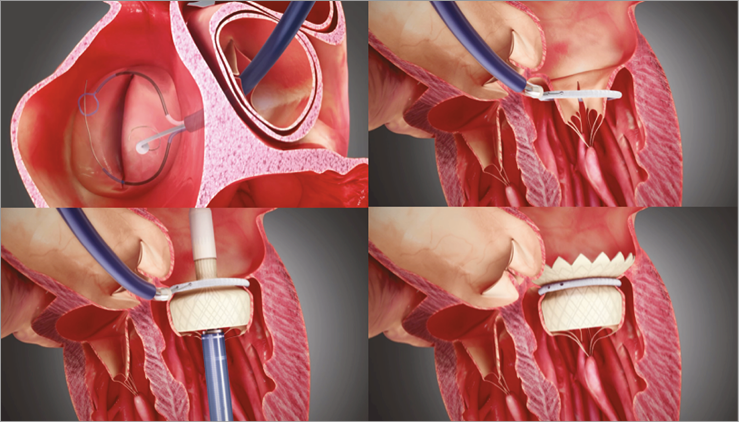
Figure 1. The LPC is advanced over a retrograde approach through the aortic valve. A guidewire loop is placed and both ends externalised. The delivery and closure of the SAI is done with a dedicated 18 Fr SAI closing catheter (SCC) hosting the SAI and the two ends of the previously placed guidewire loop. The TMV delivery catheter is inserted through either the transapical (shown above) or the transatrial access. The outflow end of the TMV is deployed distal from the SAI and the SAI positioned close to the native annulus. Then the inflow end of the TMV is deployed from the delivery catheter.
Delivery approach: Transatrial and transapical.
Device sizes/length: Not specified.
Procedural details
The current device is delivered via transatrial or transapical access. For both delivery routes, a first access is made to the femoral artery and an 18 Fr introducer sheath is positioned. This access enables the insertion of a specially designed catheter for the loop placement (LPC) and the delivery of the SAI. A second access is made either through an intercostal incision to the apex of the heart or via a right lateral mini-thoracotomy to the left atrium.
STEP 1. LOOP PLACEMENT
The LPC is advanced over a retrograde approach through the aortic valve inside the left ventricle. Two subannular tubes are deployed to reach around the subannular structure. A guidewire is caught with a snare, pulled back and externalised from the femoral access. As a result, a guidewire loop is placed, entering the femoral access travelling up to the left ventricle around the subannular apparatus and travelling back to the femoral access.
STEP 2. SAI DELIVERY
The delivery and closure of the SAI is done with a dedicated 18 Fr SAI closing catheter (SCC) hosting the SAI and the two ends of the previously placed guidewire loop. The SCC is threaded over the two ends of the guidewire loop in the left ventricle and the SAI is closed over the guidewire loop.
STEP 3. TMV DELIVERY
The TMV delivery catheter is inserted through either the transapical or the transatrial access. The catheter is advanced through the native mitral leaflets and thus through the SAI positioned around the native leaflets. The outflow end of the TMV is deployed distal from the SAI. The catheter with the TMV attached is slightly pushed (transapical)/pulled (transatrial) towards the left atrium so as to position the SAI close to the native annulus. Then the inflow end of the TMV is deployed from the delivery catheter. The catheter is closed and removed from the patient’s heart and the access to the apex/atrium is closed. The guidewire loop is removed from the SAI and SCC by pulling on one end. The SCC is removed from the femoral access along with the introducer sheath and the femoral access is closed.
Clinical data
There are no clinical data yet available.
Ongoing studies
Acute and chronic animal studies have established the safety and feasibility of the procedure. In some chronic animals, it was decided to prolong the follow-up period up to five months in order to evaluate the implants beyond the initial three-month period when anticoagulation was being administered (Figure 2). Histology showed good behaviour of the implants in vivo with adequate tissue ingrowth and limited mineralisation of the leaflets. When covered with a polyester sleeve, the SAI was completely encapsulated by ingrowing tissue creating a strong natural seal and anchor. Absence of paravalvular regurgitation, left ventricular outflow tract obstruction and significant transvalvular gradients was found in the vast majority of animal experiments.
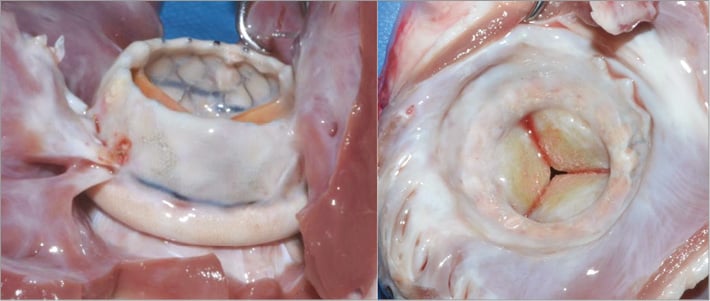
Figure 2. In chronic animal experiments up to 5 months, the HighLife transcatheter mitral valve has shown complete encapsulation by ingrowing tissue creating a strong natural seal and anchor. A large inflow (“atrial” portion) helps to seal and prevent paravalvular mitral regurgitation. The outflow (“ventricular” portion) is short and designed to avoid left ventricular outflow tract obstruction.
Unique features
The HighLife mitral transcatheter valve system consists of two components, a ring placed around the native leaflets (SAI) and a specifically designed stent with a groove placed inside the SAI. The SAI together with the native leaflets provide complete paravalvular sealing. Restrictive leaflet pathology is accommodated. Upon delivery, the valve stent centres itself within the SAI, thus facilitating potential transseptal delivery. The native valve ring is pulled by the SAI towards the valve stent, thus making sizing easier.
Potential improvements
A transseptal system is currently in development.
Conflict of interest statement
R. Lange: HighLife (consultant, equity share); N. Piazza: HighLife (consultant, equity share).

