Device description
Name and manufacturer: Tiara™ transcatheter mitral valve system; Neovasc Inc., Vancouver, BC, Canada.
Approval status: Early feasibility trial in the USA, Canada, and Belgium in 2014-2015.
Platform: The Tiara valve consists of a nitinol self-expanding frame and bovine pericardial leaflets.
Specific design: The valve is anatomically shaped to fit the asymmetric, D-shaped mitral annulus. Its atrial portion is designed to fit the atrial portion of the mitral valve annulus. Ventricular anchoring structures are designed to secure the valve onto the fibrous trigones and posterior shelf of the annulus (Figure 1, Figure 2)1,2.
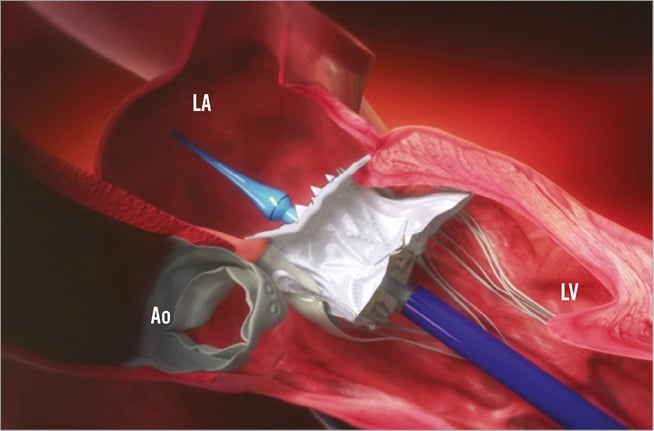
Figure 1. Design and delivery system of the Tiara valve. The valve is anatomically shaped to fit the asymmetric, D-shaped mitral annulus. Its atrial portion is designed to fit the atrial portion of the mitral valve annulus. Ventricular anchoring structures are designed to secure the valve onto the fibrous trigones and posterior shelf of the annulus. The delivery device comprises a self-dilating tip with a single turn-knob mechanism to allow controlled deployment and is designed to enter the LV apex directly without a delivery introducer sheath. Ao: aortic valve; LA: left atrium; LV: left ventricle
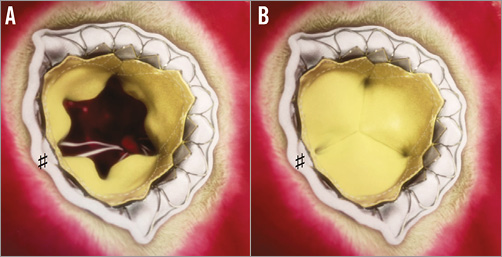
Figure 2. The Tiara valve: view from the left aatrium. A) Valve in open position; B) valve in closed position. Please note the D-shape of the prosthesis with the flat side towards the aortic side (♯).
Delivery system: The valve is loaded into a 32 Fr delivery device just before the procedure. The delivery device comprises a self-dilating tip with a single turn-knob mechanism to allow controlled deployment and is designed to enter the LV apex directly without a delivery introducer sheath (Figure 1).
Device sizes/lengths: The A-P diameter is 30-34 mm, and the lateral diameter is 35-40 mm in the 35 mm and 40 mm valves, respectively. The Tiara valve is currently available for investigational use in a single, 35 mm size. The 40 mm and 45 mm sizes are under development and will be introduced for investigational use shortly.
Procedural details
The Tiara transapical implant procedure is performed in a hybrid operating theatre under general anaesthesia with endotracheal intubation. Implantation is performed by a multidisciplinary team which includes an interventional cardiologist and cardiac surgeon, guided by simultaneous transoesophageal echocardiography (TEE) and fluoroscopy. Through a 4 cm mini-thoracotomy the LV apex is exposed, and a needle puncture of the LV apex is performed, followed by the introduction of a 0.035-inch J wire across the mitral valve into the left atrium (LA) under TEE and fluoroscopic guidance. The Tiara delivery system is introduced into the LV directly and across the mitral valve into the mid LA. The atrial portion of the Tiara is then unsheathed. Orientation and alignment of the D-shaped Tiara is performed, allowing proper fit within the native mitral annulus, with the flat aspect towards the aorta, confirmed by three-dimensional TEE. The delivery system is then pulled down to seat the atrial portion of the Tiara valve firmly onto the atrial aspect of the mitral annulus. Then the Tiara ventricular anchoring mechanisms are released from the delivery system to secure and release the prosthetic valve3,4.
Clinical data
Eight patients with severe symptomatic mitral regurgitation who were considered high risk for mitral valve surgery have been treated with the Tiara mitral valve. Six patients were implanted under special access at St Paul’s Hospital in Vancouver, Canada, five with functional mitral regurgitation and one with rheumatic mitral disease. Additionally, there have been two implants in the TIARA-I Early Feasibility Study. In seven of the eight cases, Tiara was successfully implanted as intended, resulting in a stable and well-anchored prosthetic valve with complete resolution of the patient’s mitral regurgitation. One case required conversion to a surgical valve replacement due to valve malposition. No significant transvalvular gradients, no left ventricular outflow tract obstruction, no paravalvular leaks or negative interactions with surrounding structures, including the aortic valve, have been observed.
To date there have been no mechanical failures of Tiara, including frame fractures or thrombus formation observed in clinical use.
In all, pre-discharge as well as 30-day echo showed no evidence of mitral regurgitation.
Ongoing studies
The TIARA-I is an ongoing early feasibility study. This is an international, multicentre, single-arm, prospective, safety and performance study. Medical centres in the USA, Canada and Belgium are currently enrolling patients.
The study objective is to evaluate the safety and initial performance of the Neovasc Tiara Mitral Transcatheter Heart Valve with the Tiara Transapical Delivery System.
The primary endpoint is freedom from all-cause mortality and major adverse events at 30 days from the implant procedure or hospital discharge, whichever is later.
Unique features
The structure and function of the mitral valve are far more complex than the aortic valve. This complexity poses many barriers to the development of transcatheter mitral therapies: a D-shaped annulus; the lack of a fibrous annular structure; variability of leaflet and subvalvular apparatus anatomy; and proximity to the left ventricular outflow tract, circumflex coronary artery, and coronary sinus.
The Tiara is a catheter-based self-expanding mitral bioprosthesis, specifically designed to fit the complex anatomical structure of the mitral apparatus. The valve assembly is shaped to match the natural orifice of the mitral valve to minimise the potential for obstruction of the LV outflow tract and preserve subvalvular structures. The device minimises protrusion into the LA. Tiara is fixed in place using a combination of anchors including unique active anchors which engage and seat on the fibrous trigones. The unique shape and radial expansion of the device also minimises paravalvular leak. During all stages of valve implantation until the final release and deployment, it is possible to recapture the partially deployed valve and retrieve it into the delivery catheter, reposition, and restart the implantation process.
Conflict of interest statement
S. Verheye and A. Cheung are consultants for Neovasc Inc. S. Banai is Medical Director of Neovasc Inc. M. Leon has no conflicts of interest to declare.

