Device description
Name and manufacturer: MitraClip®; Abbott Vascular, Abbott Park, IL, USA.
Approval status: CE mark in March 2008. Indication: mitral regurgitation. FDA approval in October 2013. Indication: severe symptomatic DMR too high risk for conventional mitral valve surgery as determined by the Heart Team.
Device components: Stabiliser; 24 Fr hydrophilic steerable guide catheter (SGC); dilator; clip delivery system (CDS); MitraClip device (implant): cobalt-chromium construction; polyester cover designed to promote tissue growth; (Figure 1A, Figure 1B).
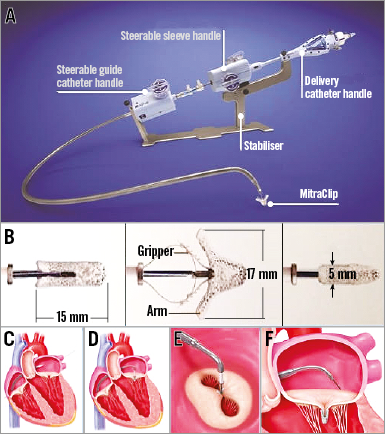
Figure 1. MitraClip device and essential phases of implantation. The MitraClip device with all components (A); details of the implant (B); different phases of the procedure showing positioning of the guiding catheter (C), alignment of the MitraClip (D), double orifice after clip implantation (E), MitraClip released with leaflets inside (F).
Specific design: The MitraClip device consists of two movable arms and grippers to grasp the mitral valve leaflets, creating a vertical line of coaptation, forming a double orifice valve.
Delivery/approach system: Percutaneous femoral venous access; requires successful transseptal puncture.
Device size: One size (Figure 1B). Possibility of multiple implants.
Procedural details
The procedure (Moving image 1) is performed under general anaesthesia, which allows for continuous transoesophageal echocardiography (TEE) to visualise the valve leaflets, the site of regurgitation (Moving image 2) and the interatrial septum. Via a transfemoral venous access, the interatrial septum is punctured at the interatrial fossa to position the SGC in the left atrium (Figure 1C). The CDS is then introduced through the SGC to orient the clip perpendicular to the mitral valve leaflets’ line of coaptation (Figure 1D). The mitral valve leaflets are grasped (Moving image 3) between the corresponding arm and gripper to result in a reduction in mitral regurgitation (MR), coaptation of the leaflets and the creation of a double orifice valve (Figure 1E).
MR is assessed throughout the entire procedure using real-time TEE (2D and/or 3D) to confirm optimal positioning and sufficient reduction in MR (Figure 1F, Moving image 4).
Clinical data
The MitraClip system is the most investigated device for percutaneous mitral valve repair with more than 25,000 patients treated.
The Endovascular Valve Edge-to-Edge Repair (EVEREST II) randomised, controlled trial evaluated the safety and effectiveness of the MitraClip compared with mitral valve (MV) surgery (repair or replacement)1. The primary safety endpoint was the incidence of major adverse events (MAEs) at 30 days; the primary efficacy endpoint was freedom from death, surgery for mitral valve dysfunction, or grade ≥3+ MR at 12-month follow-up. DMR and FMR were present in 74% and 26%, respectively. Acute device success, defined as residual MR ≤2+ after the procedure, was achieved in 77% of the patients. The study achieved the primary efficacy non-inferiority endpoint. MAEs were significantly lower in the device group (15% vs. 48%), mainly driven by the requirement of blood transfusions ≥2 units. At five-year follow-up, MV surgery to treat residual MR was needed in the device group more than in the surgical group (25.7% vs. 7.5%, respectively), driven by an increased incidence of MV surgery in the first months after MitraClip implantation. Mortality was similar in both groups at five-year follow-up (18.8% vs. 21.0%).
Once MitraClip therapy was introduced in Europe and operators gained experience in this technique, a significant number of MitraClip registries demonstrated high rates of procedural success and favourable short-term outcomes2-6. A pooled analysis of 351 patients in the EVEREST II High Risk Registry and the REALISM (Real World Expanded Multi-center Study of the MitraClip System) Continued Access Study demonstrated an implantation success of 96%5. These patients were all considered high risk for surgery (estimated STS >12%). Most patients in this cohort had FMR (70%), which reflects the major indication for MitraClip in Europe currently. Eighty-six percent of surviving patients experienced MR reduction to ≤2+ with significant reduction in LV volume at 12 months, and durable MR reduction at 24 months (84%). In addition, 83% of patients were classified as NYHA functional Class I/II at 12 months, with improvements in physical and mental components according to the SF-36 quality of life questionnaire, and annual hospitalisation rates for heart failure reduced from 0.79% pre-procedure to 0.41% post-procedure.
ACCESS-EU (MitraClip Therapy Economic and Clinical Outcomes Study Europe) is another multicentre registry with a total of 567 patients at 14 European sites, which demonstrated an implant success rate of 99.6%4. Thirty-day and one-year mortality rates were 3.4% and 19%, respectively. By one year, open mitral valve surgery was necessary in 6.3% of patients, 3.4% of patients required a second MitraClip procedure to treat residual MR, and the incidence of 3 to 4+ MR was 21%. Among one-year survivors, 71% were in NYHA functional Class I/II with improvements in six-minute walk test and quality of life scores. Similar findings were demonstrated in the European Sentinel Registry, in which most patients treated were elderly, had multiple comorbidities, and were high-risk surgical candidates3.
Several small studies suggest a benefit even in critically diseased patients. A retrospective multicentre analysis suggested a clear clinical benefit in 50 patients with an LV ejection fraction of ≤25%, demonstrating a six-month survival rate of 81.2% and improved functional class and LV reverse remodelling7. In the European PERMIT-CARE (Percutaneous Mitral Valve Repair in Cardiac Resynchronization Therapy) trial, 51 severely symptomatic cardiac resynchronisation therapy non-responders with significant FMR underwent MitraClip therapy, revealing significant MR reduction, LV reverse remodelling, and improved functional class with a 30-day mortality of 4.2%8. Data from the GRASP (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation) registry6 have shown clinical benefits in severe symptomatic FMR patients with concomitant moderate/severe tricuspid regurgitation (TR) after MitraClip treatment (i.e., improvement in MR, TR, and NYHA functional class). However, baseline moderate/severe TR remained an independent predictor for death and re-hospitalisation for heart failure at 12 months9. A GRASP substudy demonstrated that patients who fulfilled the EVEREST criteria and those who did not fulfil these criteria showed similar safety at 30-day follow-up and efficacy up to 12-month follow-up after MitraClip implantation10. Furthermore, both groups revealed statistically significant LV reverse remodelling at 12 months, but the between-group baseline differences were sustained. This study therefore suggests the potential possibility of expanding the indications for MitraClip implantation beyond the initial criteria proposed by the EVEREST study.
Ongoing studies
The COAPT (Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy) Trial for High Surgical Risk, conducted in the USA, and the RESHAPE 2 (Randomized Study of the MitraClip Device in Heart Failure Patients With Clinically Significant Functional Mitral Regurgitation) trial, conducted in Europe, investigate the effectiveness of MitraClip in patients with FMR beyond optimal medical therapy and CRT if indicated. The MATTERHORN (Mitral Valve Reconstruction for Advanced Insufficiency of Functional or Ischemic Origin) trial will compare MitraClip therapy and reconstructive MV surgery in patients with FMR. The results of these trials will clarify the role of the MitraClip in FMR. The HIRIDE trial will assess the safety and efficacy of MitraClip therapy as compared to MV surgery in high-risk patients with degenerative MR. All these trials will help to define who derives benefit from this promising technique of transcatheter mitral valve reconstruction.
Unique features
Percutaneous heart beating procedure with real-time positioning and repositioning to optimise MR reduction.
Potential improvements
Increased length of the arms and independent arms should facilitate grasping in difficult anatomies.
Conflict of interest statement
C. Grasso is a proctor for Abbott Vascular. S. Baldus has received lecture fees and research support from Abbott Vascular.
Online data supplement
Moving image 1. MitraClip procedure animation.
Moving image 2. Mitral regurgitation before MitraClip implantation. 2D echocardiography showing a flail due to chordae rupture resulting in a severe mitral regurgitation.
Moving image 3. Grasping phase (2D echocardiography LVOT view). The leaflets lean onto the opened MitraClip that is gently retracted towards the atrium. The grippers are then dropped and the clip is closed to fix the leaflets.
Moving image 4. Mitral regurgitation after MitraClip implantation. 2D echocardiography showing mild residual mitral regurgitation after three-clip implantation (compare with Moving image 2).
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. MitraClip procedure animation.
Moving image 2. Mitral regurgitation before MitraClip implantation. 2D echocardiography showing a flail due to chordae rupture resulting in a severe mitral regurgitation.
Moving image 3. Grasping phase (2D echocardiography LVOT view). The leaflets lean onto the opened MitraClip that is gently retracted towards the atrium. The grippers are then dropped and the clip is closed to fix the leaflets.
Moving image 4. Mitral regurgitation after MitraClip implantation. 2D echocardiography showing mild residual mitral regurgitation after three-clip implantation (compare with Moving image 2).

