Abstract
Aims: Unlike most diagnostic procedures, the MitraClip® therapy requires precise positioning of transseptal access to ensure a successful procedure. Radiofrequency-based transseptal puncture has been developed to reduce complications and improve precision of septal access. We report our experience utilising surgical diathermy-based transseptal puncture for MitraClip implantation.
Methods and results: Between October 2008 and April 2010, 72 patients underwent MitraClip therapy. Diathermy-assisted transseptal access was performed in 66 patients, under echocardiographic guidance, by manual contact of the diathermy blade with the Brockenbrough needle at the groin. Rate of successful puncture, time from femoral vein puncture to transseptal access and rate of complications were analysed. Diathermy-assisted puncture was successful in all cases. Time from femoral vein access to transseptal puncture was 16±19min. There was one suboptimal septal puncture position (too low), and there was one coronary artery air embolism. There were no cases of intraprocedural pericardial effusion or arrhythmias.
Conclusions: Surgical diathermy-based transseptal puncture may be a safe and effective alternative to either conventional or RF-based septal crossing. It improves precision of the septal access and may reduce the risk of bleeding complications. This technique is now routinely used at our institution for all MitraClip procedures.
Introduction
Transcatheter structural heart interventions often involve a transseptal approach to enter the left side of the heart. Transseptal puncture is a well-established procedure for diagnostic and therapeutic catheterisation1-4. However, it is still considered a delicate manoeuvre, carrying substantial risks if not well performed. Fluoroscopic guidance is usually adequate when transseptal puncture is performed for standard diagnostic procedures, electrophysiological mapping, ablation procedures, and mitral valvuloplasty. However, guidance by transoesophageal echocardiography (TEE) is becoming more popular to guide atrial septal puncture precisely in structural heart interventions.
Precision of the puncture is necessary not only to reduce the risk of complications, but also to facilitate introduction of delivery systems into the left atrium in an optimal position for device implantation. This is very well exemplified by the MitraClip (Abbott Vascular Inc., Menlo Park, CA, USA) therapy, where precise location of the transseptal puncture is mandatory to ensure a successful and expeditious procedure.
Radiofrequency (RF) based transseptal puncture has been developed in an attempt to reduce complications and improve the precision of septal puncture5-13. We developed a simple and affordable solution to perform RF puncture of the interatrial septum using standard surgical diathermy equipment and we report our experience utilising routine diathermy-based transseptal puncture for MitraClip implantation.
Methods
Between October 2008 and April 2010, 72 consecutive patients underwent MitraClip implantation at our institution. All patients had severe mitral regurgitation. Diathermy-assisted transseptal puncture was used in the last 66 patients, while a standard transseptal needle puncture was used in the first six patients. The method of action of the surgical diathermy mediated septal puncture is illustrated in Figure 1. When the surgical electrocautery is activated, an electric current is generated from the tip of the cautery blade that is fully transmitted to the tip of the Brockenbrough needle (acting as a pinpoint electrode since the rest of the needle is insulated by the plastic Mullin’s sheath and dilator), and travels towards the dispersive electrode on the patient’s leg.
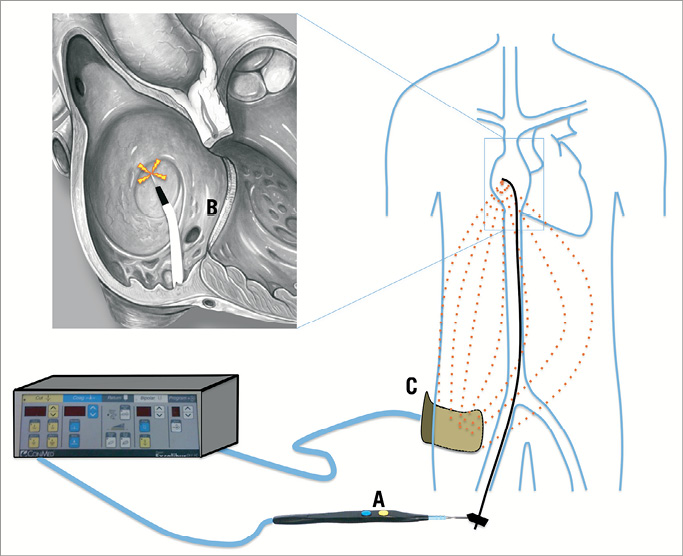
Figure 1. The method of action of the electrocautery-mediated transseptal perforation. When the surgical electrocautery is activated, a current is generated from the tip of the cautery blade that is fully transmitted to the tip of the Brockenbrough needle (the rest of the needle is insulated by the plastic Mullin’s sheath and dilator), and travels towards the dispersive electrode on the patient’s leg. Energy is dissipated in the body, and concentrated at the tip of the needle, creating heat and vaporising the tissue to allow tissue penetration.
Although the MitraClip procedure has been extensively described elsewhere, we briefly describe the most important steps of the procedure, with special regard to the transseptal puncture.
MitraClip implantation is performed under general anaesthesia. The patient is positioned on the table as usual. The electrical plate of the diathermy (dispersive electrode) is placed on the lateral side of the thigh or leg (Figure 2A). TEE guidance is used throughout the entire procedure to guide transseptal puncture and subsequent clip implantation. Three-dimensional echocardiography is rarely used for guidance. Transseptal puncture is a critical step of the procedure. In order to function properly, the MitraClip delivery system should cross the septum at an appropriate height from the annular level, according to leaflet anatomy and function. The transseptal puncture is performed in the superior and posterior-mid aspect of the fossa ovalis in most patients. The aim is to cross the septum at a distance from the mitral annulus between 3.5 and 4 cm. This optimal height is required for proper alignment of the clip delivery system and to allow enough trajectory of the clip towards the leaflet for proper grasping and leaflet insertion in the clip (Figure 3C).
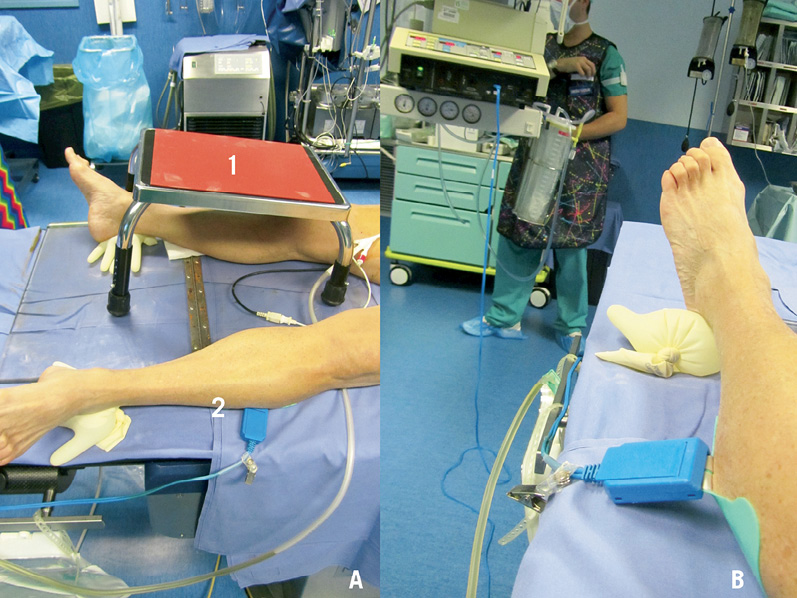
Figure 2. The room set-up. A) The caudal end of the catheterisation table. The feet are spread and supported by surgical gloves filled with water to avoid tissue compression. The inclined table for the MitraClip delivery system metallic support is positioned over the right leg (1) and the dispersive electrode is positioned on the left leg. B) The dispersive electrode is connected to the Excalibur™ diathermia generator. The “cut” function is set at 30W in pure cut mode.
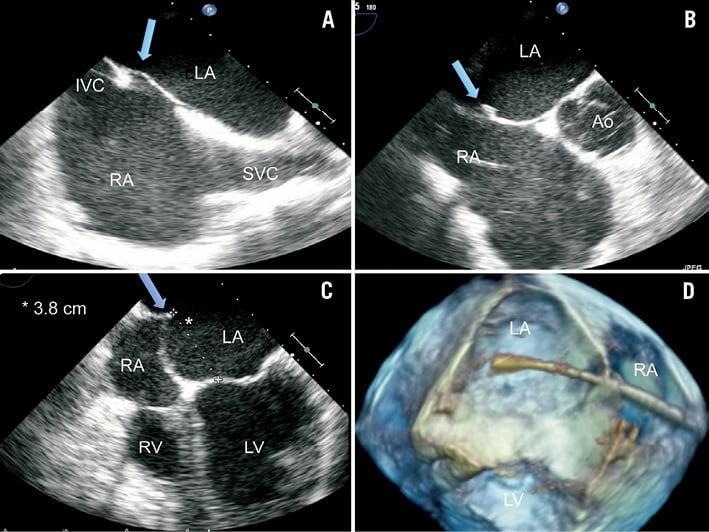
Figure 3. Main TEE images prior to transseptal puncture for MitraClip. Multiple views of the fossa ovalis prior to transseptal puncture (A to C). The arrows indicate the location of the tenting. A) Bicaval view at 2-D TEE. The needle is tenting the septum in the superior-mid portion of the fossa ovalis. B) Anterior-posterior position of the tenting is assessed at short axis at the base TEE view. The aim is to puncture sufficiently posterior to gain space between the puncture and the aortic root (anterior). C) Before septal puncture, the distance between the plane of the tenting and the annular plane is measured in the TEE four-chamber view. An ideal working distance between 3.5 and 4 cm is required in most cases: it is 3.8 cm in the example in the Figure. D) Live 3-D four-chamber reconstruction showing the high position of the transseptal crossing of the delivery system (Moving image 1).
Since the procedure is TEE-guided, a pigtail catheter is not routinely placed in the aortic root, as in the case of transseptal puncture by solely fluoroscopic guidance. A 10 Fr sheath is inserted in the femoral vein and a 0.032” J-tipped guidewire is advanced into the superior vena cava. An 8 Fr Mullin’s sheath with introducer (Medtronic Inc,, Minneapolis, MN, USA) is then advanced over the wire and its tip is placed well above the cavo-atrial junction. The Brockenbrough transseptal needle is then introduced into the Mullin’s dilator, keeping the sharp end of the needle within the dilator, but almost at the tip of it. The needle is oriented at about 45° medially and posteriorly (at five o’clock in relation to the patient) and it is pulled back under TEE and fluoroscopic guidance until tenting of the fossa ovalis is observed in the TEE “bicaval view”. Whenever possible, the tenting should aim at the superior-mid or mid portion of the fossa ovalis, in order to achieve enough height (Figure 3A). The bicaval view is obtained in the upper oesophageal position, at a 90° angle with the multiplane probe.
When the tenting position in the bicaval view is considered satisfactory, the anterior-posterior position of the tip of the needle is evaluated in the short axis at the base TEE view (Figure 3B). The latter view is usually obtained by keeping the probe in the same position as for the bicaval view while setting the TEE multiplane angle to 30°. The tenting should be posterior enough to stop the puncture being too close (anterior) to the aortic wall and to prevent the MitraClip delivery system “hugging” the aorta and being distorted by it. In case of functional disease, the puncture site is often more anterior. Finally, the distance between the plane of the transseptal puncture and the annular plane is measured in the TEE 4-chamber view (Figure 3C). Overall, the ideal distance should be between 3.5 and 4 cm, although higher puncture could be necessary for degenerative mitral regurgitation (MR) and lower puncture for functional MR due to the different level of the coaptation in the two aetiologies. A higher puncture is usually obtained by a more posterior tenting. When the ideal position of the tenting is identified, the septum is punctured using RF energy delivered with the surgical electrocautery system over the transseptal needle. Prior to delivering energy, the metal tip of the transseptal needle is slightly advanced in order to be exposed beyond the Mullin’s dilator tip. This manoeuvre is done very gently to avoid inadvertent movement of the whole transseptal system. If the needle is still in the Mullin’s dilator and thus has no direct contact with the septum, the plastic tip of the dilator will insulate the metal from the septal tissue. If the needle is advanced too far out of the dilator, energy can be dispersed with less efficacy and higher risk of charring, clotting and bubble formation (but in this case these events usually occur on the right side of the heart). Then the operator quickly touches (less than one second) the metal needle at the groin (Figure 4A) with the diathermy pencil blade while pressing on the “cut” button. The diathermy generator should be set at 30-70 watts in pure cut mode. A lower power setting (30W) is attempted first; if the puncture is ineffective, the power is increased to 50W and eventually to 70W. Any surgical electrocautery can be used: we used an Excalibur model (Conmed Linvatec Inc., Largo, FL, USA). If the needle is outside the tip of the Mullin’s dilator, the contact with the fossa ovalis closes the electric circuit of the diathermia and RF energy is delivered cutting the tissue (Figure 1). This step of the procedure is guided by TEE in the view that best shows the tip of the needle tenting the septum (usually the short axis at the base) as seen in Figure 4B. A small quantity of microbubbles visible in the left atrium is useful to confirm a successful puncture (Figure 4B to 4E). The bubbles are created by vaporisation, and are of small size. In case the septum is not crossed, microbubbles are either not seen or remain on the right side of the atrium. It is crucial that the energy delivery time is as short as possible (a fraction of a second) to avoid an excessive amount of bubbles in the left atrium, clot formation and charring of tissue with formation of embolic debris. Following septal crossing, the Mullin’s sheath and dilator are slightly rotated counter-clockwise (particularly when on the fluoroscopic AP view, the tip is oriented to the right of the patient) to avoid advancing the transseptal system towards the roof of the atrium. Keeping the needle stable, the Mullin’s sheath and dilator are advanced about 1 cm. The needle is then advanced as well, but without exiting from the sheath, in order to support the Mullin’s sheath and avoid kinking while the entire system is advanced towards the pulmonary veins. The needle and dilator are removed and an exchange length 0.035” Amplatz Super Stiff 1 cm-long J-tip wire (Boston Scientific Inc., Natick, MA, USA) is advanced into the left atrium. The Amplatz wire is preferably advanced into one of the pulmonary veins or, alternatively, it can be pre-shaped with a wide curve and positioned in the left atrium. The wire will then support the insertion of the guide catheter, which is equipped with an echo-visible tip to confirm septal crossing. The MitraClip implant procedure is then completed with the insertion of one or more clip delivery systems and clip implantation, as described in detail elsewhere14. All patients were transferred to an intensive care unit for a minimum of six hours. A transthoracic echocardiogram was performed routinely in all patients six to 12 hours following the procedure and on a daily basis thereafter.
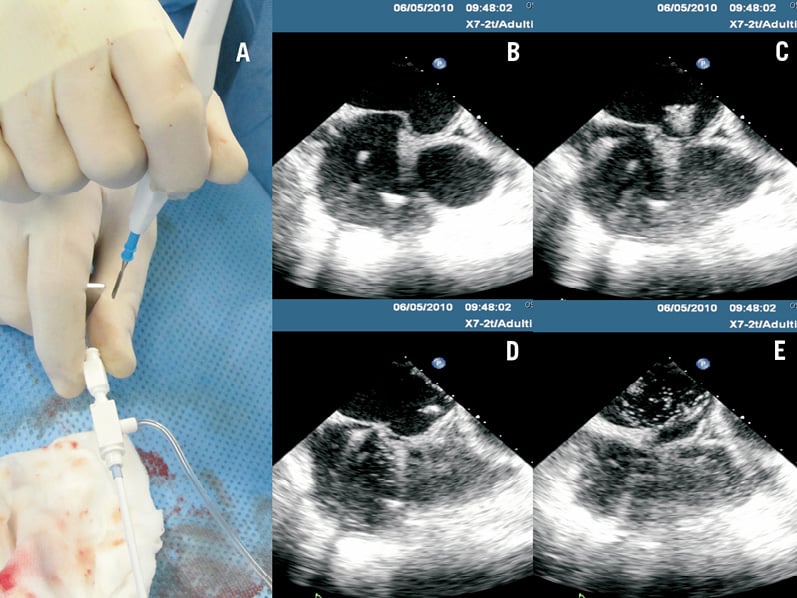
Figure 4. Diathermy-assisted septal perforation. Image in panel A shows the manual contact of the diathermia pencil blade with the transseptal needle at the groin. Panels B to E show frame-by-frame imaging of the septal perforation witnessed by evidence of microbubbles in the left atrium, and release of the tenting (Moving image 2).
All patients provided informed consent for both the procedure and subsequent data collection and analysis for research purposes. Data was collected prospectively in a dedicated database and analysed with the JMP (SAS Institute Inc., Cary, NC, USA) statistical software. Continuous variables data are reported as mean±standard deviations.
The authors had full access to, and take full responsibility for, the integrity of the data. All authors have read and agreed to the manuscript as written.
Results
The diathermy method was attempted and performed successfully in 66 cases. Transseptal puncture was also successful in patients with unfavourable anatomical features known to be associated with unsuccessful outcome: two patients with severe chest deformity, one patient with left-sided femoral vein access, nine patients with extremely convex septum, and three patients with thick fossa ovalis (up to 8 mm in thickness). Arrhythmias were not observed and diathermy was also safely used in 31 patients with an implanted pacemaker or defibrillator. In those cases, the implanted device was left active, and did not intervene due to the very brief duration of energy delivery. Time from femoral vein access to transseptal puncture was 16±19 minutes and fluoroscopy time was 22±6 minutes. Few adverse events were observed, all of them having a minor impact on the overall procedure. In one case, the septal puncture was found to be in the wrong position (too posterior and too low), leading to advanced delivery system manipulations (expert manipulation of the antero-posterior knob and of the guide catheter) to reach the target and grasp the leaflets. However, this was most probably due to incorrect assessment of the puncture site by echo-guidance rather than by the puncture technique. In one case, a transient right coronary artery air embolism with corresponding ECG signs of ischaemia was observed. In this case, the cut button was inadvertently kept pushed down after a successful septal crossing, and we observed a production of excessive bubbles in the left atrium. The ECG normalised within a few minutes and the air embolism was not associated with a major haemodynamic compromise. There were no acute pericardial effusions during the procedures, while one patient with severe congestive heart failure developed a moderate effusion 24 hours after the procedure, which required delayed pericardiocentesis and multiple aspirations of serous fluid for three days. No patient suffered from burns or other events potentially related to the use of diathermy15.
A MitraClip was successfully implanted in all but two patients who required surgical revision due to leaflet rupture and to clip entanglement, respectively. These complications were not related to the transseptal puncture, but to poor imaging guidance and inexperience in the initial phase of the transcatheter mitral repair programme.
Discussion
Our experience shows that RF-assisted transseptal puncture can be safely and effectively performed using a conventional surgical diathermy system in the challenging setting of the MitraClip therapy. Although this method has been previously described to assist with challenging anatomies, and as a bailout of conventional transseptal needle puncture, we routinely adopt this method during MitraClip therapy in order to improve precision of the puncture site and obtain an optimal placement of the delivery system.
Fluoroscopic guided transseptal puncture was first described by Ross16, refined by Brockenbrough4 and Mullins17. More recently, safety of the procedure has been improved with the addition of intracardiac echocardiography18 and TEE19. Transseptal puncture is sometimes considered a complex and high-risk procedure as, even in the hands of highly skilled operators, cardiac perforation occurs in as many as 0.9-5.4% of cases17,20-28. This potentially lethal complication has been associated with patients with very small or large atria, dilated aortic roots, thoracic spine deformities, and prior atrial septal defect closure29. Echocardiographic guidance is used to reduce this risk and to improve the precision of the puncture to facilitate more complex and advanced structural interventions such as left atrial appendage closure for chronic atrial fibrillation30, and percutaneous mitral or aortic valve balloon valvuloplasty31.
In MitraClip procedures, the location of transseptal puncture is crucial and influences the overall outcome of the procedure. When the site of transseptal puncture is not ideal, multiple adjustments need to be undertaken with the delivery system, and the procedure becomes longer, more complex and could be associated with reduced efficacy and lower safety. Quite often, the puncture is located at the edges of the fossa ovalis, towards the Waterston’s groove. Thus, TEE guidance is mandatory to determine the ideal puncture site. Using the diathermy method, septal crossing occurs without any further manipulation of the needle/Mullin’s sheath, thus minimising the risk of inadvertent cranial sliding of the system and missing the ideal puncture site, a problem which often occurs with the conventional transseptal needle technique. This is more often encountered when puncturing a thick septum or in the case of a grossly convex septum. In this case, as an example, when adopting the conventional method, the only way to cross the septum is by approaching it from the lower half, a location that is usually not ideal for the MitraClip procedure. If a more cranial tenting is attempted with a convex septum, the risk of slipping cranially is very high when advancing the needle, due to the almost parallel direction between the needle and the septal plane.
The method we described is a cost-effective alternative to commercially available RF transseptal devices7,9-12, and it is used by several operators performing MitraClip implantation.
There are previous published reports of commercially available RF transseptal devices and conventional surgical diathermy utilised in challenging septal anatomy or as a bailout after failure of conventional needle puncture. However, this is the first report of the routine application of diathermy-assisted puncture during MitraClip procedures, where the precise location of septal puncture is directly related to the ease and successful performance of the procedure.
Knecht et al have described a method to deliver unipolar RF by manual contact of an ablation catheter and the proximal extremity of the needle at the groin of the patient. In all cases this method was used as a bailout of multiple attempts of septal needle puncture32. The use of conventional surgical equipment to deliver RF through the tip of a conventional transseptal needle has been described by Gordon et al to create atrial communication in hypoplastic left heart syndrome with intact atrial septum33. A similar technique has been used by others34,35, but mainly in the field of electrophysiology, and usually only to challenge difficult anatomies, rather than to obtain a precise location of the puncture, as in our experience. Indeed, RF septal perforation is emerging as a safer method than conventional needle puncture. Most of the currently available data relates to the use of a commercial RF transseptal catheter (Baylis Medical Company Inc., Montreal, QC, Canada). Smelley et al have described a preliminary experience with the use of this RF catheter in 35 consecutive patients undergoing left-sided catheter ablation8, successful in all but one patient who underwent conventional puncture. Fromentin et al reported a prospective comparison of the RF device with conventional puncture in 148 consecutive patients who underwent a total of 241 transseptal procedures. All procedures were guided by TEE. One tamponade occurred at the end of procedure in a patient using the RF needle, and one interatrial septum dissection with aortic root haematoma occurred in the conventional group11. Winkle et al evaluated 1,550 AF ablations in 1,167 patients and compared 975 conventional transseptal punctures to 575 done using a RF electrode10. The first group showed a higher rate of failure to cross the atrial septum compared to the RF electrode (12/975 [1.23%] vs. 1/575 [0.17%]; p=0.039). In addition, the RF puncture was associated with a lower rate of pericardial tamponade (0/ 575 [0%] vs. 9/975 [0.92%], p=0.031). In all reports, the authors support the use of the RF catheter to reduce the rate of complications and facilitate septal crossing in challenging anatomies.
Although a direct comparison of our method to the commercial RF needle was not the aim of this study, our data are in line with those reported in the above-mentioned studies, thus confirming the safety and efficacy of an echo-guided transseptal puncture with a surgical diathermy set-up. In addition, since surgical diathermy generators are widely available, this method can be applied in every catheterisation laboratory to improve outcomes of transseptal puncture. We noticed that short duration of energy delivery is usually sufficient to puncture the septum, and longer delivery should be avoided to prevent bubble formation and charring. Arrhythmias were never observed during the energy delivery, since the Brockenbrough needle was shielded by the Mullin’s plastic sheath and only the relatively electrically inert fossa was in contact with the electrical field. This method appears to be a safe and effective alternative to either conventional or RF based septal puncture for MitraClip procedures. It improves precision of septal access and may reduce the risk of bleeding complications. We now routinely use this approach for septal access in all MitraClip procedures at our institution.
Acknowledgements
We acknowledge A. Guidotti, BS, for his great help in collecting data and images, and in coordinating the work.
Conflict of interest statement
F. Maisano is consultant for Abbott Vascular. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. A short video of the transseptal steps: 1. First step is done in bicaval view, the needle moves caudally (from right to left on the screen) until it falls into the fossa. 2. Second step is performed in short axis at the base view, to visualise the distance of the needle from the aorta (on the anterior-posterior axis). 3. Finally, the height of the tip of the needle from the annular level is assessed in 4 chamber views.
Moving image 2. The transseptal needle position is assessed through the three steps shown in Moving image 1. To prevent any unintended movement of the needle from the ideal location, septal crossing is achieved by rapid administration of RF with a surgical diathermy pencil on the transseptal needle (as described in the text). The administration of RF is followed by bubble formation in the left atrium and crossing of the needle tip with release of the septal tenting.

