A MitraClip (Abbott Vascular, Santa Clara, CA, USA) implantation is a one-of-a-kind procedure involving the integration of sophisticated catheter technology and advanced imaging guidance. The implantation is fully guided by a combination of live echocardiography and fluoroscopy; proper imaging is fundamental to replicate the surgical technique with a robotic-like catheter1.
Imaging is indispensable in the overall process of MitraClip therapy, from screening to follow-up. During the procedure, echo- and fluoroscopic imaging are crucial for a safe and effective access to the left atrium, as well as to guide the clip towards the target lesion. Echocardiography is key to prevent entanglement in the subvalvar apparatus and properly grasp the leaflets at the target. Today, most centres are using live 3-D technology during the grasping phase2. However, rather than full volumetric imaging, live biplane (X-plane) views are preferable to guide the delivery system in the three-dimensional left heart space, focusing on the target lesions in order to avoid imaging artefacts. The manuscript from Braun et al in this issue of EuroIntervention3, describes for the first time the value of on-line advanced multiplane reconstructions prior to clip release to assess mitral leaflet insertion in the MitraClip.
The ideal MitraClip implant should be effective and durable. These two conditions are often linked, but there are cases when a clip efficiently reduces the degree of mitral regurgitation in the acute phase, but fails over time. This condition is known as single leaflet attachment (SLA) or partial clip detachment. Inexperienced operators often tend to underestimate the importance of leaflet insertion assessment prior to deploying the MitraClip, while focussing on the haemodynamic improvement only. This attitude might induce higher rates of clip detachments, which have a tendency to reduce with time and the increased experience of the centre.
While complete clip embolisation is extremely rare (one case reported), SLA rate in the ACCESS-EU (Schillinger W, European Society of Cardiology, Munich August 2012, Personal communication) is about 4% at six months from the procedure, with most of the detachments occuring in the first days or weeks following the implant. Partial mobilisation with no leaflet detachment may also be responsible for recurrent mitral regurgitation (MR) following the procedure, when the leaflets slip out of one or both jaws of the clip, and leaflet mobility increases while the coaptation surface is reduced. Such events could probably be prevented by a more accurate assessment of leaflet insertion in the clip arms, as suggested in the manuscript from Braun et al.
Figure 1 shows the ideal leaflet insertion in the clip arms on an anatomical specimen. The leaflets are fully incorporated between the clip arms and the grippers. They are symmetric on both the long-axis and the short-axis plane. When leaflets are not inserted fully to the bottom of the groove between the arms and the grippers, the risk of leaflet mobilisation from the clip increases. However, a superficial echocardiographic assessment of the clip implant could not identify such a condition. As an example, a double orifice valve on a live 3-D surgical view is always visible, even when one leaflet is only partially included in the clip arm. Sometime a double orifice can be observed when one leaflet is just attached to the clip by one or more chordae. Under this circumstance, SLA might occur very early, even intraprocedurally.
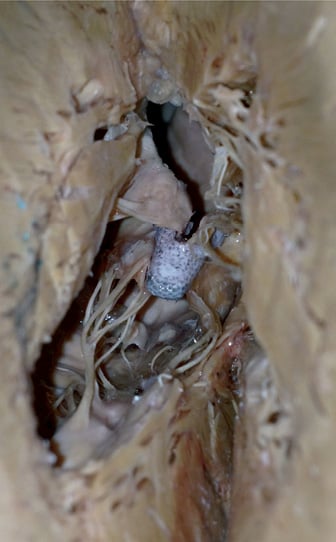
Figure 1. Anatomical specimen of a MitraClip implanted in the A2-P2 area, showing leaflet insertion.
Suboptimal leaflet insertion should be avoided because it is usually associated with recurrence of MR, it is often difficult to repair a valve with SLA with an additional clip implantation, and because it might require surgical revision. Identification of inappropriate leaflet insertion follows a sequence of steps after clip implantation. The best way to assess leaflet insertion is probably to review the echocardiographic images of the grasping. Today, most operators are recording long loops of the grasping phases and of the closure of the clip. The view typically used is the left ventricular outflow-tract long axis (LVOT) view, sometimes in association with an intercommissural view using the X-plane technology. Following grasping, the clip arms should be only partially closed to allow assessment of leaflet insertion into the jaws. Multiple views are classically used to assess insertion. In addition, or as an alternative, multiplane reconstructions could be very helpful as they have been associated with a significant decrease of SLA in the Munich experience. Leaflet insertion is separately assessed for the anterior and posterior leaflet. However, the symmetry of the implant is also important for an effective implant. Only after leaflet insertion has been thoroughly investigated is the clip closed to judge the haemodynamic effect and MR reduction. Caution should be taken in case no MR reduction is observed or when an asymmetric jet develops following clip closure. The latter event could be associated with asymmetric grasping, leaflet deformation and may deserve repositioning of the clip.
The sequence of the above-mentioned procedural steps clearly underlines that a successful MitraClip procedure involves haemodynamic improvement (by reduction of MR with no pathologic gradients) and a durable result (by means of a proper leaflet insertion in the leaflets). Advanced operators should be aware of such objectives and adopt all measures to achieve durable MR reduction; using their experience, catheter skills and advanced imaging modalities.
The growing community of MitraClip (Abbott Vascular, Menlo Park, CA, USA) implanters reacted to the zipping-by-clipping technique from Hüseyin Ince4 with a mixture of surprise, admiration and scepticism. The basic concept of the zipping technique is to implant as many clips as necessary to obtain the least degree of residual mitral regurgitation (MR) in any patient. This is very similar to the surgical approach5. We have described the edge-to-edge technique’s basic principles in different publications. Using surgical sutures, the surgeons should initially join the leaflets at the middle of the diseased segment and then extend the suture to completely obliterate the non-coapting leaflet surface6. In ischaemic MR, this often implies the obliteration of the commissure (usually the posteromedial one), while in prolapsing patients or in case of central functional MR, a double orifice is created. However, the edge-to-edge technique has been widely debated among surgeons who were not convinced by this technique.
One of the main controversies has been the risk of creating a stenotic valve. There are a large number of publications addressing this issue from the surgical perspective. We initially described the fluid-dynamics of a double orifice valve using a computational modelling approach7,8. By this method we were able to demonstrate that a double orifice valve has a haemodynamic behaviour superimposable to that of a single orifice valve with an area equal to the sum of the two orifices. In addition, the presence of an asymmetric edge-to-edge suture with orifices of different sizes does not influence global haemodynamics, and flow velocities through the orifices are similar. The most important consequence of this observation is that Doppler derived diastolic valvular properties can be obtained by probing only one of the two orifices (usually the largest). The creation of a double orifice valve is associated with a decrease in valve area. The area reduction is dependent upon three factors: the position of the edge-to-edge approximation (highest reduction when the two orifices are symmetric), the extension of the approximation (the number of clips) and the leaflet pliability9,10. The minimal acceptable resting valve area is variable according to the individual patient. However, in most patients, a total valve area greater than 2.5 cm2 rarely causes clinically relevant mitral stenosis and produces mean gradients below 5 mmHg.
These numbers should be taken as a general guideline, and small valve areas should be avoided in patients with severe pulmonary hypertension or with diastolic dysfunction. Intraprocedural monitoring of transmitral gradients (invasive and Doppler derived) and of pulmonary vein flow can be helpful to guide decision making in borderline situations. Unfortunately, the currently available MitraClip system does not allow for the reading of left atrial pressure until the clip is released and the clip delivery system is removed from the guide catheter. Therefore, invasive monitoring is not performed routinely, although some information can be achieved by the Swan-Ganz catheter. Another option, in borderline cases when there is the doubt of the risk of stenosis, is to temporarily pace the heart to increase heart rate and evoke gradients by increasing diastolic flow velocity.
The report from Paranskaya and D’Ancona et al in this issue of EuroIntervention11 describes the use of Dobutamine stress echocardiography to assess valve area and diastolic valve function under stress conditions, a technique that has been used in the past following the surgical Alfieri repair12. Similar to what is observed following surgical edge-to-edge, the valve area expands when the cardiac output increases with the effect of Dubutamine infusion, demonstrating physiological valve reserve. The inference from this observation is that resting valve area can underestimate valve area during exercise, and that valve reserve helps to keep transmitral gradients below pathological levels.
Paranskaya and D’Ancona et al, from the Ince group, have demonstrated that mitral stenosis is a rare event following MitraClip therapy and that valve reserve is maintained in most patients receiving more than two clips. It is also important to note that following such a strategy, very few patients had residual MR greater than 1/4+ at baseline as well as during Dobutamine stress echocardiography. According to these findings, MitraClip operators should aim to improve MR reduction, eventually using multiple clips more often, since the risk of creating mitral stenosis is very low and can be diagnosed intraprocedurally. In the ACCESS-EU registry, collecting data from more than 500 patients treated in several high volume centres in Europe, 60% of the patients received only one clip (Maisano, JACC in press). However, the trend is towards an increase of multiple clip implantation to achieve more effective MR reduction and reduce the risk of single leaflet attachment.
Operators should still be aware of the risk correlated to a multiple clip implantation strategy. In many occasions clip repositioning can be a better solution. As an example, when an asymmetric jet occurs following the first clip implant, the operator should rule-out an inappropriate clip implant prior to planning a second clip, and repositioning should be performed in most occasions. A second clip implant can also be inefficacious when the jet originates from a cleft distorted by a suboptimally implanted MitraClip.
MitraClip is a therapeutic tool which is amenable to individualised use according to operator skills and experience. Decision making is very complex, and relies on a strict collaboration within the heart team, not only to tailor choices to the patient’s clinical conditions and anatomical findings, but also to consider alternative options. As experience is growing, the target of MitraClip therapy should aim at more aggressive MR reduction, while preserving diastolic function, to improve clinical efficacy and reduce the need for further interventions.
Conflict of interest statement
F. Maisano is consultant for Abbott Vascular.

