Abstract
Aims: The haemodynamic effect of mitral valve (MV) repair using multiple MitraClips® (MC) has not been investigated. The aim of the study was to evaluate the stress performance of MV repair with MC.
Methods and results: Twenty consecutive patients (77±7 years, 13 men [65%]) after implantation of >2 MitraClips® were subsequently evaluated with dobutamine stress echocardiography (DSE). After MC implantation, mean transmitral pressure gradient (TPG) (3.3±0.8 mmHg vs. 4.0±0.6 mmHg; p<0.001) and mitral valve orifice area (2.9±0.3 cm2 vs. 3.9±0.4 cm2; p<0.001) were significantly increased during DSE showing a physiological behaviour effect of the MV. LVEF (41±18% vs. 46±21%; p<0.001) and systolic pulmonary artery pressure (42±11 mmHg vs. 44±12 mmHg; p=0.014) increased significantly. The degree of MR was stable during stress (p=0.68). At linear regression, only baseline peak TPG was related to stress mean TPG (p<0.001; Beta 0.816; 95% CI: 0.368-0.918).
Conclusions: MV repair using MitraClips® should be performed with the aim of maximal reduction of MR degree. MV repair using MC may not lead to pathological degrees of MV stenosis. Although the TPG is significantly increased during stress, it never reaches pathological levels and is always accompanied by a significant increase in MVOA. The degree of residual MR remains unchanged during maximal pharmacological stress.
Introduction
Percutaneous implantation of the MitraClip® (Abbott Vascular, Redwood City, CA, USA) has recently been popularised to treat patients with symptomatic severe mitral regurgitation (MR)1,2. The procedure mimics the “edge-to-edge” surgical technique developed by Alfieri et al in the early 1990s3.
Since our first MitraClip implantation, we have developed an institutional strategy with the aim of eliminating or reducing MR. In particular, we believe that the haemodynamic and anatomical basis of MR may differ greatly from patient to patient and, for this reason, we cannot expect to treat all patients using the same operative strategy and, in particular, using the same number of clips. Percutaneous mitral valve (MV) repair using multiple clips (MC) could lead, theoretically, to an excessive reduction in the MV orifice area (MVOA), resulting in a pathological increase in the transmitral pressure gradient (TPG).
To document the results of our “multiple clipping” technique we have conducted a postoperative dobutamine stress echocardiography (DSE) evaluation of our treated patients.
Methods
STUDY DESIGN
All data concerning consecutive patients treated with the MitraClip in our institution were prospectively collected in a computerised database and analysed. The indication for treatment of MR was according to current guidelines4 and was discussed in an interdisciplinary cardiology/cardiac-surgeon team. Surgical risk was assessed using the logistic EuroSCORE5 and the STS score (Society of Thoracic Surgeons) mortality risk calculation6.
PREOPERATIVE/PERIOPERATIVE EVALUATION
As part of pre-interventional screening, patients underwent echocardiography and invasive cardiac evaluation with a coronary angiogram, left ventriculography, and right heart catheterisation. All MitraClip procedures were performed as previously described2 in a hybrid operating theatre, under general anaesthesia, and using fluoroscopic and transoesophageal 2- and 3-dimensional echocardiographic guidance. The MitraClip® system included a MitraClip® device, a 24 Fr guide catheter, and a clip delivery system. A mitral valve area of <4 cm² was the limit for MitraClip® implantation7. If the reduction in MR was inadequate with one clip, further clips were placed in order to achieve maximal reduction in MR degree. The aim of the procedure was maximum reduction of MR in each case to grade ≤2.
ECHOCARDIOGRAPHY EVALUATION
Echocardiographic measurements were performed prior to the MitraClip® procedure, during the procedure, and after the procedure with a Philips iE33-xMATRIX (5 MHz transducer; Philips Healthcare, Best, The Netherlands), using standard protocols8. The DSE was carried out at a mean of 4±1 days after intervention. All patients gave their informed consent to the test.
Beta-blocking therapy was withdrawn 24 hours before the DSE. Dobutamine was administered at an initial dose of 5 µg/kg/min for five minutes, increasing to 10, 20, 30 and 40 µg/kg/min under continuous electrocardiographic and echocardiographic monitoring. Two-dimensional images and Doppler data were recorded at rest, at each stage during stress, and at recovery. Heart rate and blood pressure were recorded every two minutes. The severity of MR was graded in accordance with the American Society of Echocardiography9. Grading criteria for post-procedural MR were adapted to the quantitative assessment of the severity of MR in percutaneous MV repair as reported by Foster and co-workers10. A quantification of MR severity after MitraClip implantation was performed using Doppler colour flow mapping (regurgitant volume and regurgitant fraction) and colour jet area/left atrial area ratio. Regurgitant orifice area and vena contracta were not used as parameters for MR assessment due to lack of validation for a double-orifice valve. Measurements of left ventricular (LV) diameters were determined from a parasternal long-axis view. The systolic pulmonary artery pressure (sPAP) was derived from the maximal velocity of tricuspid Doppler tracing and assuming the right atrial pressure of 5 mmHg if the vena cava had a normal diameter or 10 mmHg if the vena cava was dilated11. Measurements of LV ejection fraction (LVEF) and volumes were performed according to the biplane Simpson’s method. The MVOA was assessed using planimetry (2-D/3-D/QULAB-Philips) only intraoperatively, and using the pressure half-time (PHT) method before implantation, intraoperatively and during DSE. The maximum and mean TPG were evaluated by planimetry of the diastolic CW-Doppler velocity signals. The degree of stenosis was acceptable by mean TPG ≤5 mmHg7. The planimetric criteria for MV stenosis were adapted according to the EAE/ASE recommendation. MS was defined as significant at rest when the valve area was <1.5 cm² or 1.7-1.8 cm², in particular in cases of unusually large patients12,13. The diastolic and systolic annular dimensions were measured in the apical four-chamber view and/or from a parasternal short-axis view. Measurements were averaged from three beats in sinus rhythm or at least five beats in case of atrial fibrillation. Measurements of all echo-Doppler examinations were performed by the same investigator. Invasive measurements of valve area and gradients after the procedure were not routinely performed and/or collected.
STATISTICAL ANALYSES
Data are presented for the group of patients submitted to MC. Continuous variables are expressed as mean ± standard deviation. Comparisons between echocardiography variables were performed including preoperative, baseline after MV repair, and DSE data. Differences between continuous variables were tested using the paired Student’s t-test. The chi-square or Fisher tests were used to compare categorical variables. Multivariable analysis by means of linear regression was performed to identify independent determinants for mean TPG at DSE. A p-value of <0.05 was considered significant. Data analysis was performed with the SPSS version 15 (SPSS Inc., Chicago, IL, USA) software package.
Results
From February 2010 to December 2011, 85 patients (mean age 78±6 years, 48 men [56.5%]) underwent MitraClip® implantation at the University Heart Center in Rostock, Germany. Successful clip placement was achieved in 82 patients (96.5%). Median total procedure time (time from puncture to closure of the femoral vein) was 197±69 min and median time for device implantation was 22±14 min.
MC (more than two clips) was used in 24 (28.2%) patients. Three clips were implanted in 19 patients, four clips in four patients, and five clips in one patient (median 3.0, range 3-5). Four patients were excluded from the study. In the first of these patients (implantation of three clips) with significant reduction of MR no procedural success could be confirmed due to significant mitral stenosis after releasing the last clip. The second patient (implantation of three clips) experienced a new significant MR due to clip detachment and underwent an emergency surgical treatment due to haemodynamic instability. The third patient (implantation of four clips) was very frail, had a severely impaired LVEF of 15%, and had a difficult post-procedural mobilisation. For these reasons he was not submitted to DSE. The fourth patient developed pneumonia and died of sepsis nine days after MitraClip® implantation. Twenty patients (77±7 years, 13 men [65%]) subsequently underwent DSE and represent the study group. All patients included in the study completed the DSE. Table 1 shows the baseline characteristics for all 20 patients.
CLINICAL FOLLOW-UP AND MORTALITY
No patient died during hospitalisation. Six-month mortality was 10.0% (two patients). No death was directly related to clip implantation. One patient died due to acute respiratory insufficiency (severe COPD) and one patient due to sudden cardiac death (Table 2).
Patients’ improvements in NYHA Class and MR are presented in Figure 1 and Figure 2.
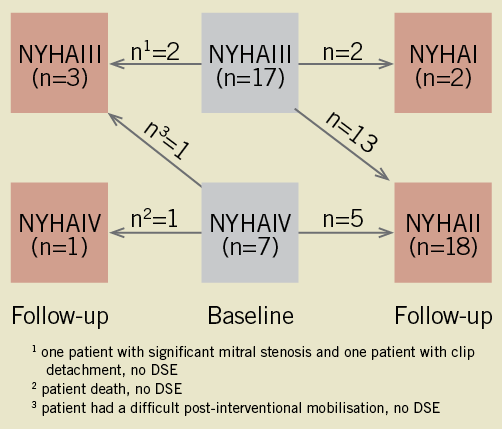
Figure 1. Improvement in New York Heart Association (NYHA) functional class at follow-up after MitraClip® implantation.
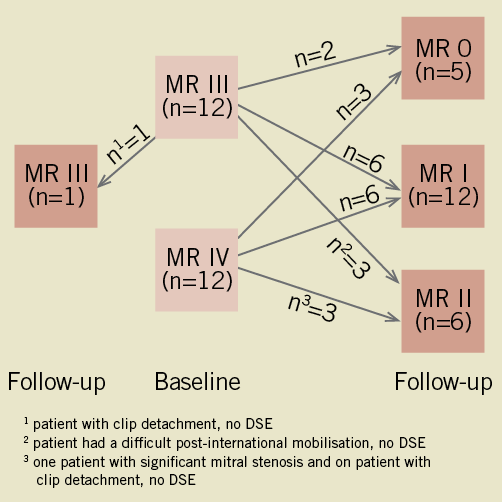
Figure 2. Improvement in mitral regurgitation at follow-up after MitraClip® implantation.
IMPACT ON ECHOCARDIOGRAPHIC PARAMETERS
At discharge, no patient had grade 3+ or 4+ MR. No patient had a significant aortic or pulmonary valve disease. Preoperatively 11 patients presented a moderate and two patients a severe tricuspid regurgitation. After MitraClip® therapy nine patients presented a moderate and no patients a severe tricuspid regurgitation. Table 3 shows the echocardiography data measured preoperatively, after MitraClip® implantation at rest and at peak during DSE. After MC, MVOA decreased significantly from 5.8±0.9 (range 4.0-7.6) to 2.9±0.3 (range 2.5-3.6) cm² (p<0.001), with a corresponding increase in the mean TPG from 2.3±0.1 (range 1.0-4.5) to 3.3±0.8 (range 1.8-5.0) mmHg (p=0.002). Furthermore, as a result of the physiological behaviour of the MV after repair, mean and peak TPG (3.3±0.8 vs. 4.0±0.6 mmHg; p<0.001 and 8.1±2.3 vs. 10.5±2.2 mmHg; p<0.001) and MVOA (2.9±0.3 vs. 3.9±0.4 cm²; p<0.001) were significantly increased during DSE. No patient developed a left ventricular outflow tract obstruction during DSE. Contractile reserve (defined by dobutamine-induced increase in LVEF ≥4%14) was present in 12 (60%) patients.
Moreover, LVEF (41±18 vs. 46±21%; p<0.001), cardiac index (2.8±0.6; p<0.001), LV end-diastolic diameter (59±9 vs. 56±9 mm; p<0.001), LV end-diastolic volume (153±56 vs. 143±53; p=0.001), LV end-systolic diameter (46±11 vs. 41±11 mm; p<0.001), LV end-systolic volume (93±42 vs. 84±40 mm; p<0.001) and sPAP (42±11 vs. 44±12 mmHg; p=0.014) were significantly different at rest after MC and during DSE.
Notice that MR degree after MitraClip® implantation and during DSE was similar (p=0.68) (Figure 3). One patient who presented no MR after MC had MR grade 1 during DSE. One patient with MR grade 1 after MC had MR grade 2 during DSE.
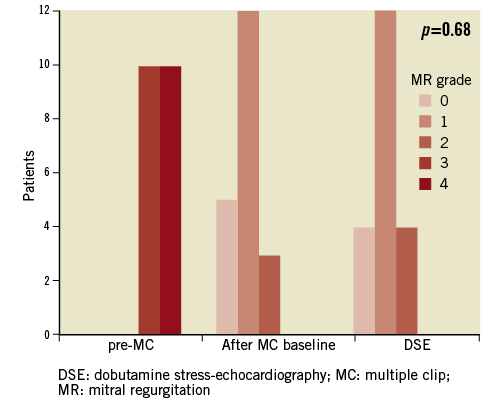
Figure 3. Mitral regurgitation grade before MitraClip® implantation, at baseline after MitraClip®, and during dobutamine stress echocardiography (DSE).
DETERMINANTS FOR MEAN TRANSMITRAL PRESSURE GRADIENT AT DOBUTAMINE STRESS ECHOCARDIOGRAPHY
A linear regression analysis model was built to chase determinants for mean TPG at DSE (Table 4). Notice that residual MR (p=0.08; Beta 0.316; 95% CI: -0.021-0.301) and peak TPG immediately after MitraClip® placement (p<0.001; Beta 0.816; 95% CI: 0.368-0.918) were the strongest independent determinants for mean TPG.
Discussion
Percutaneous treatment of MR via MitraClip® has recently been popularised to treat patients rejected for conventional surgical treatment. Although the technique is very promising, some technical aspects of MitraClip® implantation remain operator-dependent and have not been fully standardised. Dynamic evaluation after MitraClip® has, to our knowledge, never been proposed before. In fact, in the present literature there are reports mainly of one or two clips implanted in >95% of the cases with implantation of more than two clips seldom reported1,11,15. In our institution MC were implanted in 24 (28.2%) patients. In this context, the implications of multiple clips on the MV haemodynamics are particularly interesting. Our procedural success rate (96.5%) is in line with the existing literature1,2. Moreover, our results using a multiple clipping strategy confirm, both at rest and during maximal stress, the absence of iatrogenic stenosis after percutaneous correction of MR with MC. The significant increase in MVOA during DSE confirms the preserved pliability and elasticity of the valve even after MC. It has to be emphasised that MC also allowed for a constant correction of MR which in fact remained unchanged even during the most strenuous phases of stress.
Borghetti et al examined 44 patients classified in a group undergoing a quadrangular resection of posterior leaflet with mitral annuloplasty using an autologous pericardial ring (group I, n=23) and in another group undergoing MVR with a Carpentier-Edwards rigid ring (group II, n=21). Postoperative echocardiographic study did not show significant mitral regurgitation at rest or at peak exercise in any patient. Detailed analysis of this interaction showed a significant increase of LVEF in group I (p=0.005), whereas no changes were detected in group II. The velocity of transmitral blood flow increased significantly during exercise in both groups16. In another series of 27 patients, Borghetti et al evaluated the physical stress performance of MV repaired using the “edge-to-edge” Alfieri repair combined, in the majority of patients, with MV annuloplasty. A peak TPG of 17±10 mmHg was observed with pulmonary hypertension developing in 50% of patients during stress17. There is some speculation that, though effective MV competence was achieved in the majority of patients, MV repair induced impaired diastolic mitral valve dynamic during exercise conditions. Agricola et al conducted a similar study including 30 patients (27 with associated MV annuloplasty). They reported non-pathological increases in TPG and sPAP during the various phases of stress. In fact, the mean TPG (2.8±1.3 vs. 4.6±1.9 mmHg, p<0.00001), peak TPG (6.4±2.8 vs. 10.5±4.6 mmHg, p<0.00002) and systolic pulmonary artery pressure (22.8±6.1 vs. 28.2±9.9 mmHg, p<0.001) were increased, but not to pathologic levels. Planimetric valve area also increased significantly (3.2±0.6 vs. 4.3±0.7 cm2, p<0.00001)18. The results from Alfieri’s group have recently been confirmed by Hori et al19. In a comparison between patients submitted to conventional MV repair and edge-to-edge (with MV annuloplasty) the mean TPG increased significantly in both groups. Systolic PAP was also significantly elevated, but still within the accepted upper limits in both groups. The MVOA showed no significant increase in either group. At peak exercise there were no significant differences in TPG, sPAP, and MVOA between the two groups. The resting values of pulmonary arterial pressure do not necessarily reflect the actual severity of the mitral valve disease. It is known that a reduced compliance of the atrioventricular valve results in a more pronounced increase in pulmonary arterial pressure during exercise or DSE. Hence, DSE may provide the essential information in determining the severity of mitral valve stenosis in patients with previously assessed moderate mitral valve stenosis at rest due to exercise-induced symptoms20.
Our implantation strategy focuses mainly on the decision to implant as many clips as required to guarantee intraoperative optimal correction of the MR and avoid iatrogenic MV stenosis. The indication to use MC should be supported by adequate MR haemodynamics and MV anatomy. We do not believe that a “one fits all” strategy should be used to treat patients who actually present different mechanisms of MR. The decision about the number of clips to implant should be rationalised preoperatively, confirmed intraoperatively or eventually modified according to intraprocedural real-time echocardiographic monitoring. Although intraoperative real-time valve haemodynamics could be of great help and are sometimes used to take a final decision about additional clip placement, we do not have any specific data and, in fact, we did not develop a structured and routine approach to guide the final number of implanted clips. We can only state that, generally speaking, the main goal of the procedure was to achieve MV regurgitation ≤ than grade 2 with an intraoperative transmitral gradient ≤ than 5 mmHg. In any case, we cannot propose a universal algorithm that may be widely applicable to all patients. In fact, for each case good results relate predominantly to the individual operator making a decision as to when to add a further clip. In our experience, patients with narrow jets involving a limited area of the MV can and should be easily handled with one or two clips. Patients with broader and more complex jets involving a more extended area of the MV should be considered for MC. Usually, after having performed the percutaneous atriotomy, entered the left atrium, and placed the MitraClip® sheath, the first clip is placed. The clipping is started at the level of the posteromedial commissure (P3-A3), virtually to create a percutaneous commissuroplasty and to initiate a progressive approximation of the two mitral leaflets along the new coaptation line15. It is important to emphasise that patients who are nowadays referred for this experimental procedure most often present enlarged MV annular dimensions and MVOA. This is the result of global LV dilatation that leads to stretching of the MV subvalvular apparatus, consequent tethering of the MV leaflets, and deepening of the MV coaptation depth. All these conditions will eventually lead to a wide regurgitation jet extended to the entire surface of the MV. There are some basic technical tips to operate a MC procedure minimising the time expenditure and the risk of cardiac trauma and MV stenosis. After having evaluated the MV anatomy and jet shape, the decision is taken as to whether to handle the insufficiency with one or more clips. The intraoperative echocardiography should guide the decision. It should be emphasised that most often the intraoperative transoesophageal echocardiography is biased by the patient’s haemodynamic conditions during general anaesthesia. In fact, anaesthesia induction most often leads to systemic vasodilatation and indirect modification of the MR shape and entity. For this reason we operate a pharmacological stress test to increase systemic arterial pressure and to test the MV function. Intraoperative real-time 2-D and 3-D echocardiography guide the entire procedure together with fluoroscopy. The strategy of MC should be supported by real-time imaging that must document a progressive decrease in the entity of the MR and the necessity for additional clipping.
Limitations
The main limitations of the study are the limited sample size and the fact that DSE was performed only a few days after the procedure. For this reason, although we can state that no pathological TPG developed immediately after correction, we have no information concerning the follow-up stress performance of the repaired valves. It has to be remarked that, in any case, we did not document any change in the follow-up MVOA and TPG at rest.
Furthermore, inclusion of and comparison with patients implanted with fewer than two clips were not performed and would possibly have demonstrated a significant difference in stress gradients between the two groups. In reality, this was not the goal of our investigation. In fact, we aimed at determining if multiple clipping might have generated pathological gradients at rest and under maximal pharmacological stress.
Some comments should be made about the use of DSE. Although exercise echocardiography would allow the evaluation of the performance of the repaired MV in a more physiological condition, artefacts of the Doppler signals may present as a result of increased chest wall motions and respiratory rate during physical stress. Moreover, limited exercise tolerance is often present in these multimorbid patients. For these reasons, DES could be proposed as an effective alternative21. In addition, although the linear regression analysis model was significant (p=0.005), a larger number of patients is needed to make a better assessment of the true predictors of mean TPG during DES. Finally, it is known that direct planimetry is the method of choice to quantify stenosis severity, but it is not always feasible. Anatomic MVOA measured by direct planimetry has an excellent correlation with PHT-MVOA and the Gorlin formula at rest21. The MVOA is dependent on several haemodynamic parameters: left atrial pressure, stroke volume, and transmitral flow rate. Hence, measurements of mean transvalvular gradient and, indirectly, planimetry by PHT are highly rate-dependent and flow-dependent, and they have limitations in patients with calcific annular MS and impaired left ventricular compliance12,22.
Conclusions
The haemodynamic and anatomical basis of MR may differ greatly from patient to patient and, for this reason, we cannot expect to treat all patients using the same operative strategy and, in particular, using the same number of clips. This means we believe that every “clipping procedure” should be aimed at the same goal, i.e., significant reduction of MR. In order to achieve this goal, MC can be used. Although during DSE the TPG of clipped MVs is significantly increased, it never reaches pathological levels and is always accompanied by a significant increase in MVOA. Furthermore, the degree of residual MR remains unchanged in the majority of patients during maximal pharmacological stress.
Conflict of interest statement
The authors have no conflicts of interest to declare.

