
Abstract
Aims: Our aim was to evaluate the acute effects of transcatheter edge-to-edge mitral valve repair using the MitraClip device on mitral valve geometry in patients with functional mitral regurgitation (FMR).
Methods and results: Forty-two patients (age 73 years [IQ range 66.1-78.0], 55% men, 62% ischaemic FMR) with moderate-to-severe and severe FMR treated with the MitraClip were included. Three-dimensional transoesophageal echocardiography was performed prior to and immediately after MitraClip implantation. Acute changes of mitral annular and leaflet geometry were assessed with dedicated mitral modelling software. FMR less than moderate grade was achieved in 36 (86%) patients. After MitraClip implantation, the mitral annulus became more elliptical (ellipticity from 122±17% to 129±18%; p=0.04) with a non-significant reduction in anteroposterior diameter (33±6 to 32±5 mm, p=0.08). The coaptation area increased from 350 mm2 (IQ range 289-493 mm2) to 434 mm2 (IQ range 328-523 mm2, p=0.008). In particular, a larger part of the anterior mitral leaflet was included in the coaptation, leaving a smaller exposed anterior leaflet length of the A2 segment (from 27±6 mm to 25±5 mm, p<0.05) while the exposed length of the posterior leaflet (P2 level) remained unchanged (12±4 mm pre- vs. 13±4 mm post-repair, p=0.15). There was no change in total leaflet area (1,811±582 mm2 pre- vs. 1,870±506 mm2 post-repair, p=0.18). Annular height to intercommissural width ratio and tenting volume remained unchanged, suggesting no increase in leaflet stress.
Conclusions: The MitraClip device affects MV geometry in FMR patients by increasing mitral annular ellipticity and coaptation area.
Abbreviations
FMR: functional mitral regurgitation
LV: left ventricle
MVQ: mitral valve quantification
TEE: transoesophageal echocardiography
TTE: transthoracic echocardiography
Introduction
Functional mitral regurgitation (FMR) is common in heart failure patients and is associated with poor clinical outcomes1-4. Surgical mitral valve repair has proven to be beneficial, providing significant improvement in symptoms and left ventricular (LV) function5,6. However, many patients with significant FMR are not referred for or are denied surgical intervention due to a high operative risk, related to advanced age and the presence of associated comorbidities7. Several minimally invasive and transcatheter-based mitral valve repair techniques provide feasible alternative treatment options to conventional valve surgery for patients with a high operative risk. The transcatheter edge-to-edge mitral valve repair technique using the MitraClip® system (Abbott Vascular Structural Heart, Menlo Park, CA, USA) is designed to grasp the mitral valve leaflets at the middle scallops, creating a double orifice valve during diastole and maintaining closer apposition of the leaflets during systole to reduce the regurgitant volume. With more than 17,000 patients treated worldwide, the MitraClip device has been demonstrated to be a feasible and safe procedure, also in patients with FMR, and to improve symptoms8,9. The effect of the MitraClip device on the geometry and function of the mitral valve, however, remains largely unexplored. Three-dimensional (3D) transoesophageal (TEE) echocardiography allows accurate measurements of mitral valve geometry10. Accordingly, the present study aimed to evaluate the acute effects of transcatheter edge-to-edge repair on mitral annular geometry and leaflet coaptation zones of patients with FMR using 3D TEE.
Methods
PATIENT POPULATION AND DATA COLLECTION
A total of 59 patients were treated with the MitraClip device at the Leiden University Medical Centre between January 2012 and July 2014. Thorough clinical and echocardiographic evaluation was performed prior to the procedure by an interdisciplinary team of cardiac surgeons and cardiologists. Patients had symptomatic moderate-to-severe or severe mitral regurgitation and were at high risk for conventional surgical mitral valve repair, defined by a logistic EuroSCORE >20% or the presence of specific risk factors associated with excessive morbidity and mortality. Evaluation of clinical symptoms included assessment of functional capacity according to the New York Heart Association (NYHA) functional class and the six-minute walking distance test. Furthermore, a quality of life assessment was performed using the Minnesota Living With Heart Failure® questionnaire11. Transthoracic echocardiographic (TTE) assessment and TEE assessment were routinely performed before the intervention to assess left ventricular (LV) dimensions and function, mitral valve morphology and mitral regurgitation grade, and evaluation of factors that may contraindicate the procedure12. The procedure was guided with 3D TEE allowing acquisition of 3D data of the mitral valve. In the current analysis, only patients with FMR and sufficient quality of periprocedural 3D TEE data allowing geometrical analysis of the mitral valve were included (n=42).
TWO-DIMENSIONAL (2D) TRANSTHORACIC ECHOCARDIOGRAPHIC EVALUATION
Pre-procedural TTE was performed using commercially available ultrasound systems (E9 or Vivid 7; GE Norway, Horten, Norway) equipped with an M5S transducer, and 2D, M-mode and Doppler data were acquired with the patient in the left lateral decubitus position. LV dimensions and function were assessed according to the recommendations of the American Society of Echocardiography and the European Association of Echocardiography13. From the apical 4- and 2-chamber views, the LV end-diastolic and end-systolic volumes and LV ejection fraction were measured according to the biplane Simpson’s method. LV diameters were assessed at end-diastole and end-systole from the parasternal long-axis view or M-mode recordings. From the apical 2-, 3- and 4-chamber views, colour and continuous wave Doppler data of the mitral valve were acquired, and mitral regurgitation was quantitatively determined by the proximal isovelocity surface area method and by measuring the vena contracta according to current guidelines14.
3D TRANSOESOPHAGEAL ECHOCARDIOGRAPHIC DATA ACQUISITION AND EVALUATION
TEE was performed during the procedure using a commercially available ultrasound system (iE33; Philips Medical Systems, Andover, MA, USA) equipped with a fully sampled matrix-array TEE transducer (X7-2t Live 3D-TEE transducer, iE33; Philips Medical Systems) capable of acquiring both 2D and 3D images to guide implantation of the MitraClip device and to assess acute procedural success. Three-dimensional images were acquired during the intervention before and directly after implantation of the MitraClip device, using multi-beat (7-14 beats) full-volume or one-beat 3D-zoom acquisitions encompassing a pyramid volume. The multi-beat full-volume images were acquired during respiratory breath hold whenever possible to avoid stitch artefacts. In patients with atrial fibrillation, one-beat 3D-zoom acquisitions with the sector adjusted to include the mitral valve were performed. Furthermore, special care was taken to stabilise the probe during 3D data acquisition. All images were digitally stored for off-line analysis with the MVQ software (QLAB Cardiac 3DQ v.10.0; Philips Medical Systems), which allows semi-automated 3D quantification of the mitral valve geometry10. From 3D full-volume or zoomed data sets of the mitral valve, the software displays three orthogonal multiplanar reformation planes of the mitral valve at end-systole. The planes are manually aligned across the mitral annulus in a selected end-systolic frame to obtain bicommissural, outflow tract and short-axis views of the mitral valve. The bicommissural view was used to indicate the anterolateral and posteromedial points of the mitral annulus, whereas the outflow tract view was used to define the anterior and posterior points of the mitral annulus, the aortic annulus and the mitral leaflet coaptation point. The mitral leaflet commissural points were set on the short-axis plane, and the mitral leaflets and coaptation length were traced on multiple cross-sections (18-30) in the outflow tract view, orthogonal to the intercommissural direction. The 3D-rendered surgical en face view was used to identify the coaptation point correctly (Figure 1). Subsequently, the software automatically creates a 3D model of the mitral valve geometry and various measurements can be derived.
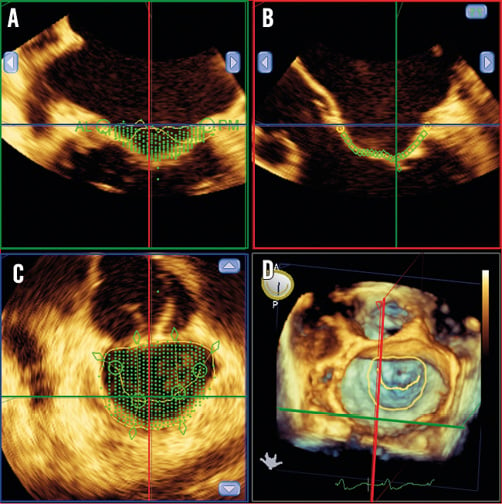
Figure 1. Identification of 3D landmarks on multiplanar reformation planes using Mitral Valve Quantification (MVQ) software. Using the multiplanar reformation planes, the MVQ software identifies the landmark points of the mitral annulus. The anterior, posterior, anterolateral and posteromedial points of the mitral annulus are identified on the 2- and 3-chamber views. The en face view provides the cross-sectional area of the mitral annulus and simultaneously the 3D full volume of the mitral valve can be visualised. A) Identification of leaflet insertion points. B) Tracing the mitral leaflets and marking the leaflet coaptation point. C) Tracing the coaptation line. D) 3D surgical view of the mitral valve with the manually traced lines visible.
The measurements of the mitral valve with the MVQ software were performed at end-systole and included anteroposterior and intercommissural annular diameters and area, and leaflet lengths, angles and areas. Mitral annular ellipticity was calculated as the ratio between the anteroposterior annular diameter and the intercommisural diameter. Furthermore, to assess the effect of the MitraClip on mitral leaflet coaptation, the coaptation length at the middle scallops (A2-P2) was measured and the coaptation area was calculated by subtracting the exposed area of the anterior and posterior mitral leaflets (i.e., the area without the coapting part of the leaflets) from the total area of the anterior and posterior leaflets (i.e., the area including the coapting part of the leaflets). Moreover, the effect of the MitraClip on leaflet stress was analysed by assessing the annular height to intercommissural width ratio and the non-planarity angle15. The non-planarity angle was determined as the angle between the anterior and posterior leaflet hinge points of the annulus to the centre of the intercommissural line. Additionally, tenting height at the A2-P2 level, tenting volume and the angle between the mitral annulus and aortic annulus were measured. Three-dimensional TEE data were analysed by two experienced observers. The intra-observer reproducibility of the anteroposterior and intercommissural diameter measurements was good, with respective intraclass correlation coefficients of 0.865 and 0.883 as well as the inter-observer reproducibility with respective intraclass correlation coefficients of 0.755 and 0.855. Intraclass correlation coefficients for overall (total) leaflet area were 0.903 (intra-observer) and 0.947 (inter-observer)16. For exposed leaflet area measurements, the intraclass correlation coefficients were 0.955 and 0.947 for intra-observer and inter-observer reproducibility, respectively. An example of mitral geometry measurements performed before and after MitraClip implantation in a patient using MVQ software is displayed in Figure 2.
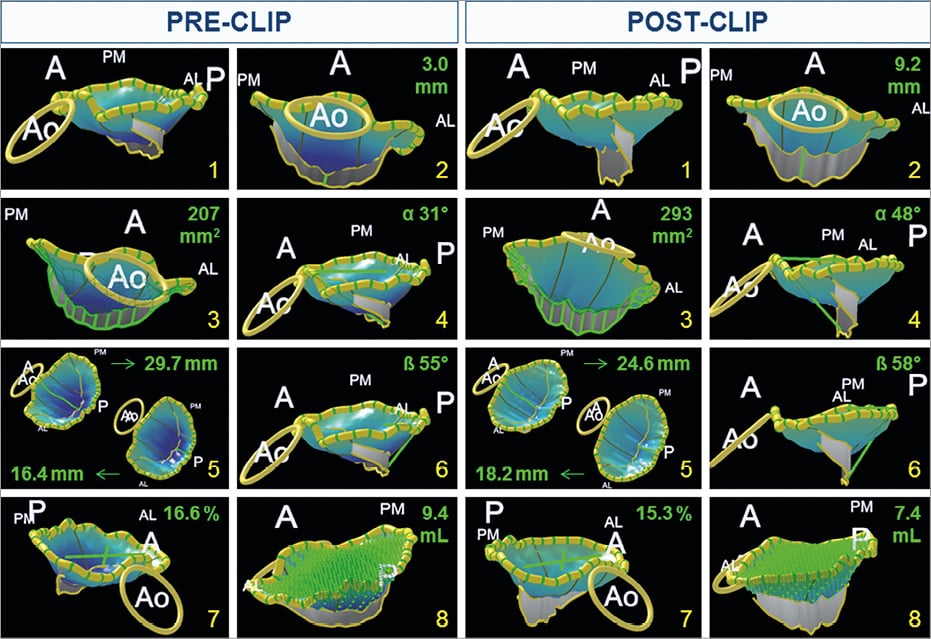
Figure 2. Example of changes in mitral valve geometry in a patient before and after MitraClip implantation with 3D model. 1) 3D model of the mitral valve. 2) Coaptation length at the A2-P2 level pre- and post-MitraClip implantation. 3) Coaptation area pre- and post-MitraClip implantation. 4) Angle between anterior leaflet and mitral annulus aorta pre- and post-MitraClip implantation. 5) Length of the anterior (A) and posterior (P) leaflet at the A2 and P2 level, respectively, pre- and post-MitraClip implantation. 6) Angle between posterior leaflet and mitral annulus pre- and post-MitraClip implantation. 7) Ellipticity. 8) Tenting volume. A: anterior; AL: anterolateral; Ao: aorta; P: posterior; PM: posteromedial
PROCEDURAL TECHNIQUE
The transcatheter edge-to-edge mitral valve intervention was performed as previously described using the MitraClip system and guided by fluoroscopy and 2D and 3D TEE12,17. In brief, after transseptal punction, the clip delivery system was advanced into the left atrium and positioned above the mitral valve plane over the origin of the regurgitant jet. The device was oriented perpendicular to the line of coaptation and the system was advanced into the LV with the arms slightly opened. After ensuring good alignment of the device (just below the regurgitant orifice on the TEE bicommissural view of the mitral valve) and simultaneously visualising the arms of the device opened in the perpendicular TEE view (120-150°, or LV outflow tract view), the system was pulled back to grasp the mitral leaflets in the arms of the clip and create a double orifice valve. The grade of MR was assessed during the procedure using colour and continuous wave Doppler echocardiography. Procedural success was defined as a reduction of MR to less than moderate grade. If needed, a second clip was placed to ensure less than moderate grade MR without significant stenosis.
STATISTICAL ANALYSIS
Distribution of the continuous data was tested by the Kolmogorov-Smirnov one-sample test and the Shapiro-Wilk test. Normally distributed continuous variables are presented as mean±standard deviation, whereas non-normally distributed variables are presented as median and interquartile range. Categorical variables are expressed as frequencies and percentages. Comparisons of mitral valve geometry pre- and post-procedure were performed using the paired Student’s t-test for normally distributed variables and the Wilcoxon signed-rank test for non-normally distributed variables. All statistical tests were two-tailed and a p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS for Windows, Version 20.0.0 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CHARACTERISTICS
Baseline demographic, clinical and echocardiographic characteristics of the patients are listed in Table 1. Moderate-to-severe FMR was observed in 48% of patients and severe FMR in 52%. Ischaemic heart failure was the most frequent underlying aetiology of heart failure (62%).
CHANGES IN 3D MITRAL VALVE GEOMETRY AFTER MITRACLIP
FMR reduction to less than moderate grade was achieved in 36 (86%) patients. End-systolic changes of mitral valve geometry post MitraClip implantation are presented in Table 2. After the procedure, the mitral annulus became more elliptical (increase in ellipticity from 122±17% to 129±17%, p<0.05) and there was an increase in both the anterior (from 27±7º to 34±8º, p<0.001) and posterior (from 49±14º to 56±10º, p=0.02) mitral leaflet angles (Figure 3). The anteroposterior mitral annular diameter decreased from 33±6 mm to 32±5 mm, although this change was not statistically significant (p=0.08). Furthermore, an increase in both coaptation length at the A2-P2 level (from 5.0±1.12 mm to 5.3±0.87 mm, p<0.001) and total coaptation area (from 215 mm2 [IQ range 164-264] to 281 mm2 [IQ range 238-386], p=0.005) was observed (Figure 4). In addition, the exposed length of the anterior mitral leaflet (i.e., excluding the coapting part of the leaflet) at the A2 level (from 27±6 mm to 25±5 mm, p=0.03) and the exposed anterior leaflet area (from 795±277 mm2 to 730±222 mm2, p=0.04) decreased while the posterior leaflet length at the P2 level and total leaflet area remained unchanged, indicating that the improvement in leaflet coaptation was due to a larger area of the anterior mitral leaflet involved in the coaptation (Figure 5). The annular height to intercommissural width ratio, non-planarity angle and tenting volume remained unchanged after MitraClip therapy, suggesting no increase in leaflet stress.
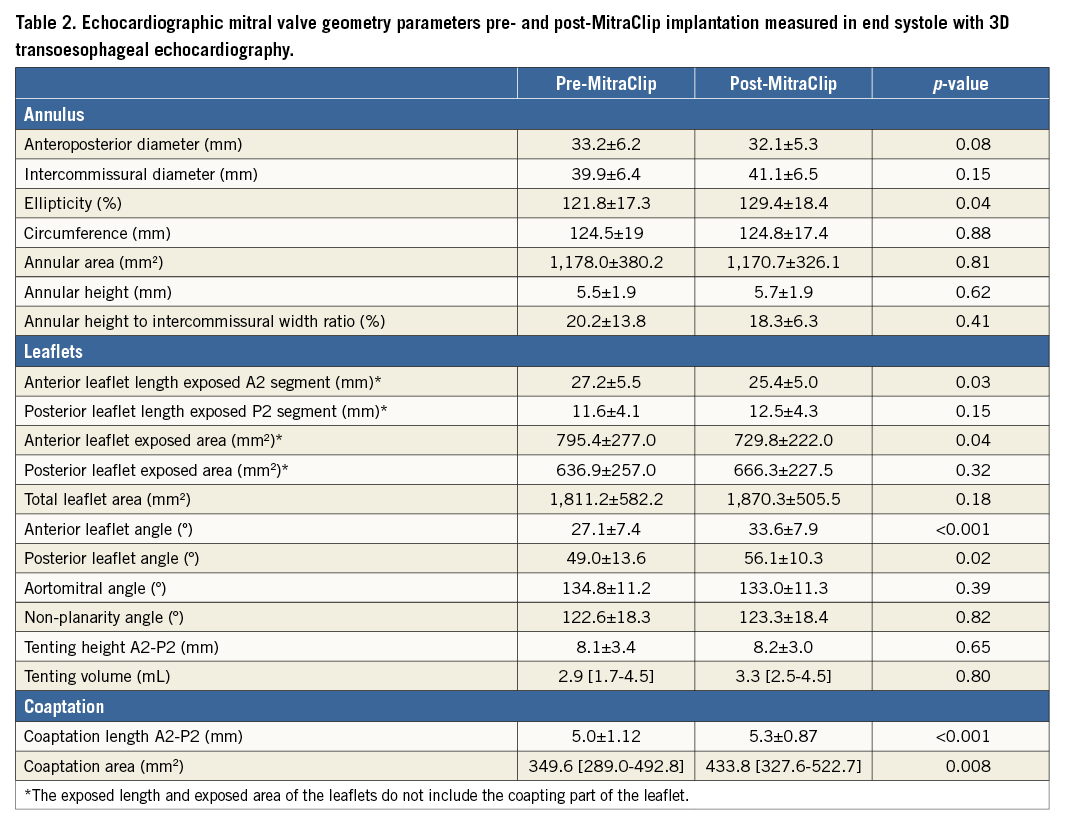
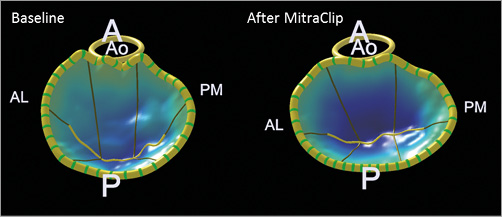
Figure 3. 3D modelling of the acute effect of the MitraClip device on mitral valve geometry. After MitraClip implantation, the anteroposterior diameter reduced from 36.5 mm to 33.5 mm, while the intercommissural diameter slightly increased from 41.5 mm to 43.7 mm and the ellipticity increased consequently from 113.7% to 130.7%. A: anterior; AL: anterolateral; Ao: aorta; P: posterior; PM: posteromedial
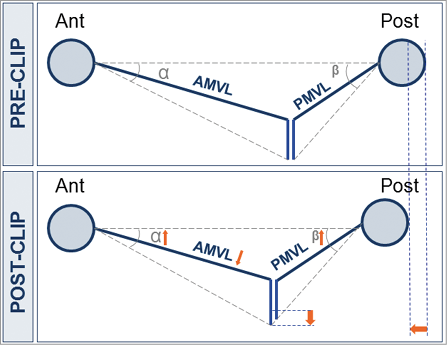
Figure 4. Schematic overview of acute effect of MitraClip device on mitral valve geometry showing that the MitraClip device leads to a decrease in exposed anterior leaflet length (AMVL) at the A2 level. Furthermore, the anteroposterior diameter tends to reduce with corresponding increases in the anterior and posterior mitral leaflet angles (α and β). AMVL: anterior mitral valve leaflet; PMVL: posterior mitral valve leaflet
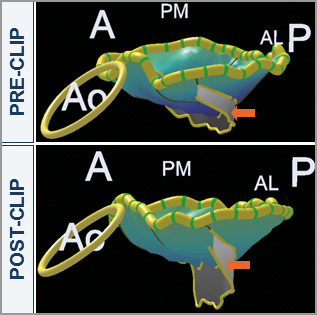
Figure 5. 3D overview of acute effect of MitraClip therapy on mitral valve geometry showing an increase in coaptation area. The long-axis view of the 3D model is shown at baseline and after MitraClip implantation with the arrow pointing to the area of coaptation. A: anterior; AL: anterolateral; Ao: aorta; P: posterior; PM: posteromedial
Discussion
The results of this study demonstrate that the percutaneous edge-to-edge mitral valve repair procedure using the MitraClip device directly affects mitral valve geometry. By grasping the anterior and posterior leaflets, the coaptation length and area of the mitral leaflets significantly increase in end-systole, with a large proportion of the anterior mitral leaflet grasped and included within the device arm while the posterior mitral annulus is anteriorly approximated.
Few studies have assessed the effect of surgical annuloplasty on mitral valve anatomy using 3D TEE. Greenhouse et al used intraoperative 3D TEE to examine the regional effects of surgical mitral annuloplasty on annular geometry and leaflet coaptation zones in patients with FMR18. A significant increase in mitral leaflet coaptation length after band annuloplasty was observed, particularly at the middle scallops of the anterior mitral leaflet (2.9±2.6 mm at baseline vs 5.5±2.5 mm post surgery, p<0.01). The posterior annulus was displaced anteriorly, leading to an increase of coaptation surface of the anterior leaflet and correction of the mitral regurgitation. Our findings are in agreement with this concept by showing that the exposed length and area of the anterior leaflet at the central scallop (i.e., the anterior leaflet length and area without the coapting part) decreased. These changes were not accompanied by changes in the total mitral leaflet area, indicating inclusion of a large proportion of the anterior leaflet into the area of coaptation. Therefore, implantation of a MitraClip device seems to affect mitral coaptation geometry similarly to surgical mitral annuloplasty, albeit through a different mechanism.
In patients with FMR treated with the MitraClip device, Schmidt et al reported significant reductions of the mitral annular area (mean difference 0.39±0.49 cm2, p<0.001) and anteroposterior annular diameter (mean difference 0.28±0.32 cm, p<0.001) as well as a significant reduction in tenting area (mean difference 0.39±0.49 cm2, p<0.001)19. The disparate results of the study by Schmidt et al and the present study may be explained by the different post-processing analysis. While Schmidt et al performed the measurements on the orthogonal multiplanar reformations of the 3D TEE volume set, in the present study the measurements were performed on 3D models that permit assessment of the saddle shape of the mitral annulus. However, the current analysis did show a change in the shape of the mitral annulus with an increase in annular ellipticity and slight decrease (not statistically significant) in anteroposterior diameter, which is consistent with previous reports19-21. Recently, Schueler et al showed that patients with FMR showed significant decreases in the anteroposterior diameter of the mitral annulus (from 4.0±0.6 cm to 3.6±0.6 cm, p<0.001), 3D mitral annulus area (from 14.4±3.9 cm2 to 12.9±3.4 cm2, p<0.001) and mitral valve sphericity index (from 0.9±0.1 to 0.8±0.1, p<0.001), whereas the intercommisural diameter remained unchanged21. Interestingly, these changes were not observed in patients with degenerative mitral regurgitation. Furthermore, patients in whom the anteroposterior diameter acutely reduced ≥6.4% had superior clinical response to MitraClip therapy after six months of follow-up compared with patients showing less acute annulus diameter reduction. However, an acute reduction in the anteroposterior diameter after MitraClip therapy would indicate significant traction on the mitral leaflets to reduce the distance between the anterior and posterior annulus. This high leaflet stress could affect the durability of the procedure. To assess leaflet stress, we analysed the annular height to intercommissural width ratio. It has been shown that mechanical leaflet stress is minimal when the saddle shape of the mitral annulus is preserved (i.e., annular height to intercommissural width ratio <15-20%)15. In the present study, the annular height to intercommissural width ratio did not significantly reduce after MitraClip implantation, suggesting no acute increase in leaflet stress.
Understanding the effects of the MitraClip on the geometry of the mitral valve will eventually help to identify patients who will benefit most from this procedure. Currently available 3D TEE data post-processing software has provided further insight into how this device affects the geometry of the mitral valve and which changes are associated with sustained reduction in FMR at mid-term follow-up21. Additional advances in post-processing software or development of novel computational simulation software22 may help to improve the implantation strategy along with new developments of the device that may improve the acute and long-term outcomes of this technique.
Limitations
There are several limitations that should be acknowledged. First, the study population was rather small. By recording both the pre- and post-procedural views during anaesthesia we aimed to create a similar haemodynamic status for the pre- and post-procedural measurements of mitral annular geometry. However, the effects of the anaesthetics on patients’ haemodynamic status must be considered. Furthermore, the clips caused shadowing artefacts and limited the evaluation of the coaptation point. By tracing the coaptation point in the body of the MitraClip after the procedure, which is the point where the leaflets are grasped by the clip, we aimed to reduce the effect of the artefacts on the outcomes.
Conclusions
Percutaneous MitraClip therapy affects mitral valve geometry in FMR patients by increasing coaptation length and area due to a larger contribution of the anterior mitral leaflet to coaptation after the procedure.
| Impact on daily practice Evaluating the effects of MitraClip on 3-dimensional mitral valve geometry is important to understand its therapeutic efficacy and durability. The present study showed that MitraClip increases the coaptation length and area due to a larger contribution of the anterior mitral leaflet into the coaptation. Furthermore, the anteroposterior diameter tends to reduce with corresponding increases in the anterior and posterior mitral leaflet angles. Subsequent iterations of the device or development of newer systems may consider these findings to improve the efficacy of the repair. Although the effects of these geometrical changes on long-term durability were not evaluated in the present study, future investigations may shed light on this topic and help in the design of novel devices. |
Funding
The Department of Cardiology of the Leiden University Medical Center has received research grants from GE Healthcare, St. Jude Medical, Medtronic, Boston Scientific, Edwards Lifesciences and Biotronik.
Conflict of interest statement
V. Delgado has received consulting fees from St. Jude Medical and Medtronic and speaker’s fees from Abbott Vascular. P. Debonnaire is supported by a Sadra Medical Research Grant (Boston Scientific), holds a European Association of Cardiovascular Imaging Research Grant for 2013, receives speaker’s fees from Abbott Vascular and is a faculty member of Abbott Vascular Crossroads. No specific financial support for this work is involved. The other authors have no conflicts of interest to declare.

