CASE SUMMARY
BACKGROUND: A 71-year-old woman affected by idiopathic dilated cardiomyopathy with normal coronary arteries and permanent atrial fibrillation was found to have severe mitral regurgitation at transthoracic echocardiography (TTE), due to annular dilatation and restricted motion of the posterior leaflet. Because of poor quality of life, high functional class (NYHA Class III) and the high risk of surgery, the patient agreed to undergo the implantation of a MitraClip® device. During the procedure, the transoesophageal echocardiographic (TEE) images were of a poor quality since the view of the mitral valve in the mid-oesophageal and transgastric projections did not accurately show the valve leaflets and the convergence area of the regurgitation at colour Doppler, which is indispensable for the correct positioning of the clip.
INVESTIGATION: Physical examination, transthoracic echocardiography, transoesophageal echocardiography.
DIAGNOSIS: Severe mitral regurgitation suitable for MitraClip™ implantation.
MANAGEMENT: Transthoracic, and not transoesophageal, echocardiography approach during MitraClip™ procedure.
KEYWORDS: Mitral regurgitation, MitraClip™ device, transoesophageal echocardiography, transthoracic echocardiography
PRESENTATION OF THE CASE
A 71-year-old woman has been followed up for several years at the Cardiovascular Department of the Ospedali Riuniti of Bergamo, affected by an idiopathic dilated cardiomyopathy with normal coronary arteries (angiography performed in 2002), permanent atrial fibrillation, NYHA functional Class III, excess weight, previous thrombophlebitis of the left lower limb, total thyroidectomy for multinodular thyroid goiter, melanoma treated with interferon in 1995, bilateral coxarthrosis and depressive syndrome.
On physical examination, the patient had jugular turgor, hepatojugular reflux, hepatomegaly, no peripheral oedema, arrhythmic cardiac tones, a 3/6 intensity systolic murmur on the precordium, attenuated vesicular murmur, BMI 29 kg/m2. Blood tests showed only anaemia (Hb 10.3 g/dl and Hct 32.7%). The brain natriuretic peptide (BNP) levels ranged from 199 to 644 ng/L in the last two years. The electrocardiogram showed atrial fibrillation with an average ventricular rate of 46/min, left intraventricular conduction delay (QRS duration 120 ms). The transthoracic echocardiography (TTE) showed biatrial dilation (left atrium area 40 cm2, volume 39.6 cm3, right atrium area 33 cm2), left ventricular dilation (EDD/ESD 68/59 mm, LVDV/LVSV 185/122 ml, LVDVI/LVSVI 101/67 ml/m2), normal thickness (IVS/PW 9/9 mm), severe left ventricular systolic dysfunction related to diffuse hypokinesia (left ventricular ejection fraction 28%), with an increase in left ventricular filling pressure (restrictive diastolic function with a transmitral E-wave DT 117 ms, and an E/e’ ratio >15), severe mitral regurgitation (effective regurgitant orifice 30 mm², regurgitant volume 41 ml, vena contracta 6 mm, ratio colour jet area/left atrial area 59%), with central jet origin, dilated ring (annulus 39 mm), and restricted motion of the posterior leaflet (with a reduced coaptation length of the leaflets). The planimetric mitral valve area in the parasternal short-axis view was 7 cm2. Other impaired parameters were a mild aortic insufficiency, a dilated right ventricle (diameter 37 mm) with a reduced longitudinal contractility (TAPSE 16 mm, annular S’ TDI velocity 0.07 m/s), a dilated inferior vena cava with reduced inspiratory collapse (from 28 mm to 23 mm), moderate tricuspid regurgitation, severe pulmonary hypertension (estimated pulmonary artery systolic pressure 50±15 mmHg). The transoesophageal echocardiography (TEE) confirmed that the mechanism of mitral regurgitation was a mitral annular dilatation and a restricted motion of the posterior leaflet with a consequent reduced coaptation length (3 mm) of the leaflets and a coaptation depth of 5 mm. The convergence area of the regurgitant flow at colour Doppler was very extensive, involving the central part of the valve (A2-P2), but also P1 and P3.
Because of poor quality of life, high functional class (NYHA Class III) and the high risk of surgery the patient agreed to undergo the implantation of a MitraClip™ device (Abbott Vascular, Santa Clara, CA, USA)1. On the day of intervention we induced a general anaesthesia, performed an orotracheal intubation and placed a Swan-Ganz catheter, which confirmed the severe pulmonary hypertension and high left ventricular filling pressures. The TEE images were of poor quality even though performed by cardiologists with expertise in the methodology. Since the outpatient TEE was performed with the patient positioned in the left lateral decubitus, it was decided to place a radio-transparent material under the right hemithorax of the patient, trying to reproduce conditions of analysis satisfyingly similar to those of the ambulatory setting. In this way it was possible to guide the operator during the transseptal puncture and the advancement of the guiding catheter and the delivery system towards the mitral valve. Unfortunately, the view of the mitral valve in the mid-oesophageal and transgastric projections did not accurately show the valve leaflets and the convergence area of the regurgitation at colour Doppler, which is indispensable for the correct positioning of the clip (Figure 1). We first tried to retract and replace the TEE probe, with no beneficial effect in obtaining a good view of the mitral valve. We also made an attempt to use a nasogastric tube to improve the TEE images, again with no satisfying results2,3.
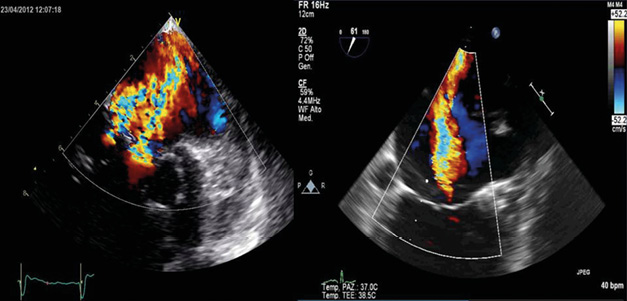
Figure 1. TEE view of the mitral valve in the mid-oesophageal and transgastric projections did not accurately show the valve leaflets and the convergence area of the regurgitation at colour Doppler.
How would I treat?
THE INVITED EXPERTS’ OPINION
The authors present a planned MitraClip™ implantation in a patient in whom intra-procedure transoesophageal echo (TEE) was severely limited. The most common approach to device navigation for MitraClip placement relies on TEE, and increasingly on 3D-TEE4,5. While TEE imaging guidance is taught to new operators and is well understood by experienced implanters, it is possible to consider each of the major steps of the procedure and how alternative imaging modalities might be used. Transseptal puncture, movement of the delivery system through the left atrium without getting stuck on the walls, appendage, or Coumadin ridge, coarse alignment over the centre of the line of coaptation, fine alignment over the regurgitant jet origin, and leaflet grasping/grasp assessment must all be considered. TEE, angiography and fluoroscopy, possibly intracardiac echo, and transthoracic echo (TTE) might be used in combination to overcome the limitations of TEE in this case. If TEE were not adequate to guide the transseptal puncture ICE might be used. After the transseptal puncture, left atrial angiograms in AP and shallow RAO projections can be used to define atrial and mitral valve landmarks and facilitate many of the initial steps of the procedure. The Clip Delivery System can be introduced into the LA and the MitraClip extended from the guide catheter to its initial working position entirely with the use of the fluoro landmarks, since the upper and lateral borders of the LA are well defined by the angiogram. Similarly, coarse navigation to the line of coaptation can be accomplished with fluoro. While the TEE is clearly limited in this case, the images shown with the case description make it clear that TEE would be of some help. Fine adjustment of the position of the MitraClip over the jet origin can be done with short-axis TTE or the parasternal LAX/apical 3-chamber/apical 2-chamber views. The TTE details reported for this patient suggest she can be reasonably imaged despite her elevated BMI. The step of rotating the clip to be perpendicular over the line of coaptation is currently done most commonly with 3D-TEE, but can be approximated with fluoro. In a shallow RAO view the clip should be seen from the side. TTE might be used to verify perpendicularity, as it was early in the development of this procedure. Intracardiac 2D echo (ICE 2D) is not ideal for verifying perpendicularity but soon to be developed ICE 3D and colour may prove to be useful for clip perpendicularity imaging and clip deployment. Leaflet grasping might be the toughest procedure step to assess, but hopefully some information would come from TEE. If the MR jet could not be located accurately, the strategy of using the first clip to stabilise the leaflets or leave part of the jet medial or lateral could be employed. A second clip could then be deployed to grasp the remaining jet using primarily fluoro. These techniques require considerable experience with the MitraClip procedure.
Conflict of interest statement
T. Feldman is a consultant to and receives research grants from Abbott. The other author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
This a very challenging case of a 71-year-old woman with idiopathic dilated cardiomyopathy, severe left ventricular systolic dysfunction (left ventricular ejection fraction 28%), with a dilated ring (annulus 39 mm; planimetric mitral valve area in the parasternal short-axis view of 7 cm²), and restricted motion of the posterior leaflet (with a reduced coaptation length of the leaflets) leading to a severe functional mitral regurgitation (FMR). Supported by Franzen et al, current data suggest that severe FMR in patients with end-stage heart failure and marked LV dysfunction can be reduced by MitraClip implantation and that successful therapy promotes clinical benefit at six months in the majority of cases6. However, a considerable number of patients (as in this case) with FMR present with extensive annulus dilatation, accounting for minimal vertical leaflet coaptation that might preclude them from the standard single-clip approach. In this context, septal to lateral zipping of the tethered mitral valve, our so-called “zipping by clipping technique”, should be considered as an alternative strategy7, aiming at a significant reduction of MR, because residual mitral valve regurgitation after percutaneous mitral valve repair with the MitraClip system is a risk factor for adverse one-year outcome8, demonstrated first by our group and confirmed by the Swiss registry9.
Our implantation strategy focuses mainly on the decision to implant as many clips as required to guarantee intraoperative optimal correction of the MR and avoid iatrogenic MV stenosis. The indication to use multiple clips (MC) should be supported by adequate MR haemodynamics and MV anatomy. We do not believe that a “one fits all” strategy should be used to treat patients who actually present different mechanisms of MR. The main goal of the procedure is to achieve MV regurgitation ≤ grade 2 with an intraoperative transmitral gradient ≤5 mmHg. The zipping is started at the level of the posteromedial commissure (P3-A3), effectively to create a percutaneous commissuroplasty and to initiate a progressive approximation of the two mitral leaflets along the new coaptation line. It is important to emphasise that patients who are nowadays referred for this experimental procedure most often present enlarged MV annular dimensions and MV orifice area. This is the result of global LV dilatation that leads to stretching of the MV subvalvular apparatus, consequent tethering of the MV leaflets, and deepening of the MV coaptation depth. All these conditions will eventually lead to a wide regurgitation jet extending to the entire surface of the MV, as in this patient.
In terms of the suboptimal imaging of this case, it highlights the increasing need for more detailed information on 3D cardiac structures. Merging data from different non-invasive imaging modalities (MDCT, MRI, x-ray and echocardiography [2D and 3D]) provides enhanced functional and anatomical information in real time and these are under investigation.
In any case, we cannot propose a universal algorithm that may be widely applicable to all patients. Patients such as the one in this case with broader and more complex jets involving a more extended area of the MV should be considered for the zipping technique.
Conflict of interest statement
H. Ince is a proctor for Abbott. The other authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
We decided to try to combine TTE and TEE. After the first attempt at positioning the clip, while the TEE showed a fair result, the TTE very clearly showed a partial grasping of the posterior leaflet with moderate inferomedial residual regurgitation originating from A3-P3 evident in the parasternal short-axis and apical 2-chamber views (Figure 2). The clip was therefore reopened, withdrawn and subsequently reoriented and advanced enough to perform a wider grasping on the rear flap. After the second attempt, the TTE parasternal short-axis view showed a centrally positioned clip, fully grasping the rear edge, with an 8-shaped symmetrical loop of the mitral valve in diastole (Figure 3) and a medial mild to moderate residual regurgitation, confirmed by apical projections. The procedure was therefore completed with the release of the clip without complications. The following fluoroscopy and TTE documented a correct movement of the clip and a residual regurgitation of mild degree. At the end of the procedure the control TTE, better than TEE, documented the good result of the clip positioning (Figure 4).
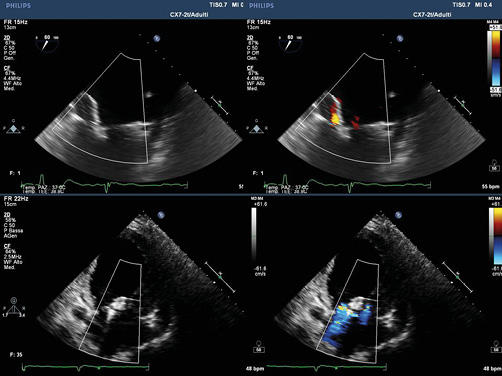
Figure 2. TTE very clearly showed a partial grasping of the posterior leaflet with moderate inferomedial residual regurgitation originating from A3-P3 evident in the parasternal short-axis and apical 2-chamber views.
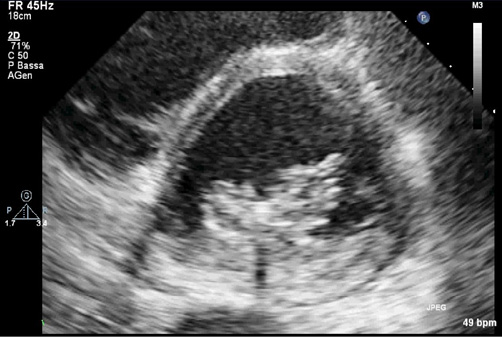
Figure 3. TTE parasternal short-axis view showed a centrally positioned clip, fully grasping the rear edge, with an 8-shaped symmetrical loop of the mitral valve in diastole.
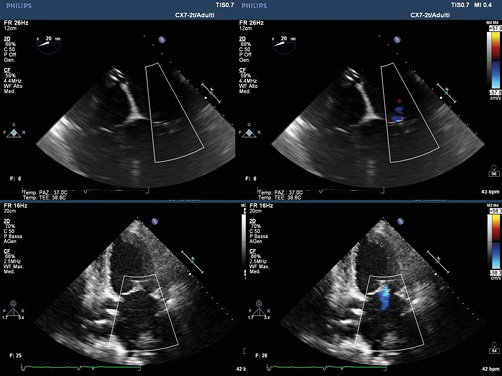
Figure 4. TTE, better than TEE, documented the good result of the clip positioning.
The patient, after a period of observation in the intensive care unit of about 24 hours, was transferred to the cardiology ward. The pre-discharge echocardiogram confirmed the success of the procedure with mild residual mitral regurgitation.
To date, of the 8,000 patients in the world10-12 treated with a MitraClip™, to our knowledge this is the first case demonstrating the ability to monitor the MitraClip™ implant process combining a TTE and TEE approach. When the latter is unable to provide images of good quality, necessary for the correct positioning of the device, TTE might be successfully used.
Conflict of interest statement
The authors have no conflicts of interest to declare.

