Mitral transcatheter edge-to-edge repair (M-TEER) has been established as a treatment approach in symptomatic severe mitral regurgitation (MR) patients. However, even after successful M-TEER, some patients develop recurrent MR over time. Electrosurgical Laceration and Stabilization of Clip devices (ELASTA-Clip) is a novel technique for cutting a mitral tissue bridge, enabling subsequent transcatheter mitral valve implantation (TMVI)1. Very few ELASTA-Clip cases have been reported, and to date, this technique has only been applied in patients with one, or a maximum of two, M-TEER devices.
A 67-year-old female presented with highly symptomatic, recurrent severe MR 2.5 years after successful MitraClip M-TEER with three devices (MR reduced to 2+, mean gradient 3.0 mmHg). Given her symptomatic burden and high surgical risk, we decided to perform a triple ELASTA-Clip procedure with subsequent TMVI.
Transapical surgical access was prepared, and a 24 Fr DrySeal sheath (W.L. Gore & Associates) was placed in the femoral vein for dual guiding sheath insertion. Two 8.5 Fr steerable Agilis NxT (Abbott Vascular) guiding sheaths were placed transseptally. A Swan-Ganz balloon catheter was used to traverse the medial orifice of the clipped mitral valve through the first Agilis. The second sheath was placed and flexed towards the lateral orifice, while the soft unexpanded part of the inserted 27-45 EN Snare system (Merit Medical), guided by a JR4 catheter, was used to traverse the lateral ostium. After positioning an Astato 0.014 coronary guidewire (Asahi Intecc) via the medial system, the Swan-Ganz was exchanged for a JR4 guide catheter. The Astato wire was pointed towards and advanced to the lateral snare, which, after exposing the basket, was used to snare the Astato wire.
The guidewire was then focally denuded and kinked externally to create a so called “flying-V” configuration. By pulling the snared Astato wire through the lateral Agilis/multipurpose system, the flying V was positioned on the ventricular side of the clips. Using echocardiographic and fluoroscopic guidance, this flying V was placed directly adjacent to the tissue bridge of the three MitraClips. It is key to encircle the anterior mitral valve leaflet of the clip package to ensure that the clip package stays connected to the posterior mitral valve leaflets after burning and can be pushed into a posterior pocket by the prosthesis. A 70 W continuous duty current was applied to the flying V wire with light traction on the lateral and medial catheters. After successful laceration, with all three MitraClips still attached to the posterior mitral valve leaflet, transapical TMVI using a Tendyne (Abbott Vascular) prosthesis was performed. Adequate positioning resulted in proper TMVI function (mean gradient 4.0 mmHg, no paravalvular leakage) (Figure 1, Moving image 1). The patient was discharged, reported immediate symptomatic benefit, and after six months of follow-up, showed a well-functioning TMVI prosthesis (mean gradient 3.6 mmHg, no paravalvular leakage).
Recurrent MR after M-TEER is a challenging scenario, as interventional treatment options seem limited, especially, in the complex anatomical setting of three implanted M-TEER devices. However, the presented experience highlights that even in more complex settings, full transcatheter restoration of proper mitral valve function is feasible after ELASTA-Clip. As the rate of recurrent MR after M-TEER rises following the increasing number of implants, standardisation of procedures such as ELASTA-Clip, eventually by means of dedicated devices, seems to be needed.
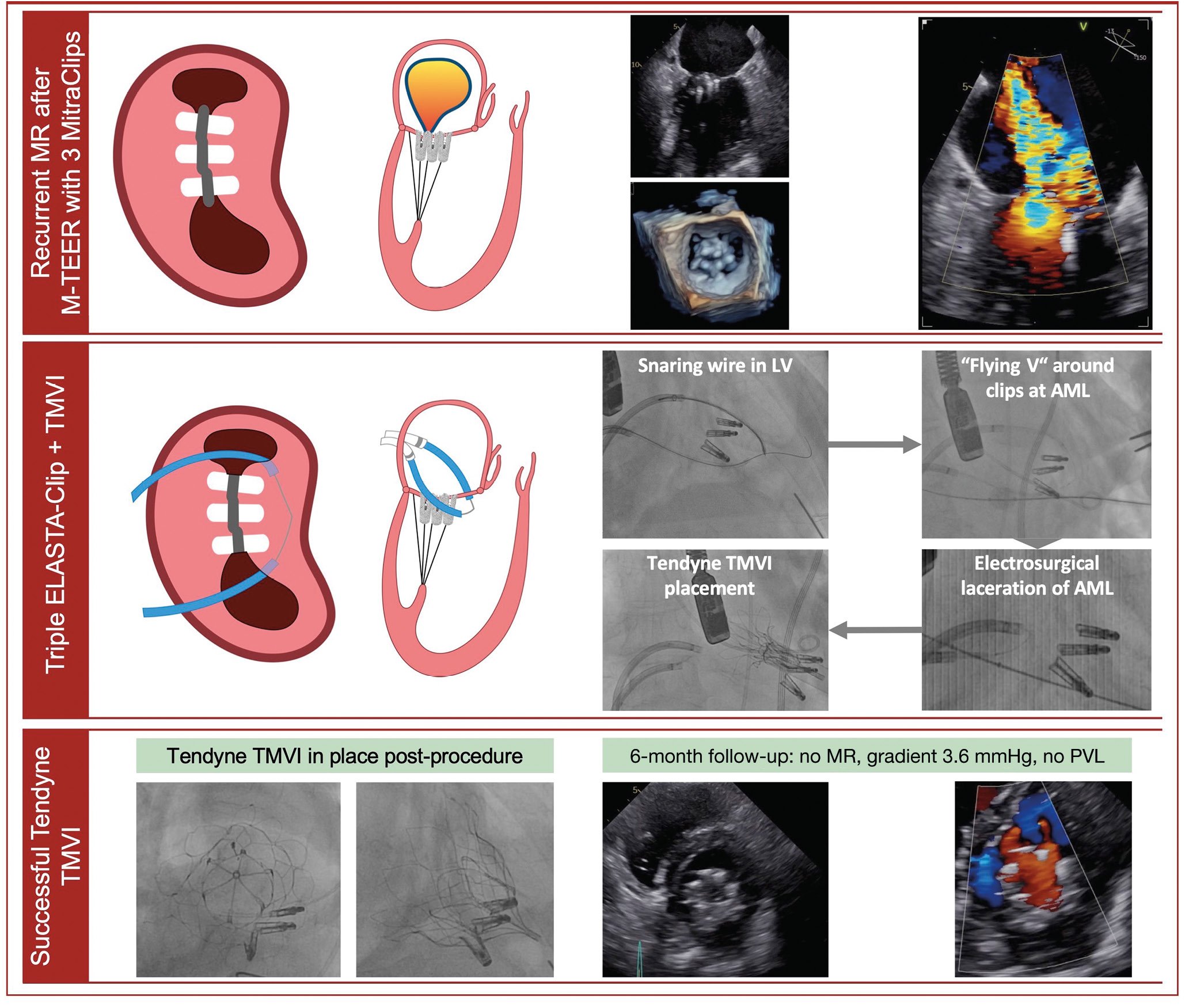
Figure 1. ELASTA-Clip electrosurgical laceration of three MitraClip M-TEER devices and subsequent TMVI. AML: anterior mitral valve leaflet; LV: left ventricle; MR: mitral regurgitation; M-TEER: mitral transcatheter edge-to-edge repair; PVL: paravalvular leakage; TMVI: transcatheter mitral valve implantation
Conflict of interest statement
J. Curio has received research grant support from Boston Scientific. J. Khan is a proctor for Edwards Lifesciences and Medtronic; a consultant for Transmural Systems; and an inventor on patents, assigned to the National Institutes of Health, on leaflet laceration technology. S. Baldus has received lecture fees from JenaValve; and lecture and speaker fees from Edwards Lifesciences. M. Adam has received grants and personal fees from Medtronic; and personal fees from JenaValve, Edwards Lifesciences, and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Preprocedural imaging, ELASTA-Clip of three M-TEER MitraClip devices, subsequent Tendyne TMVI and 6-month follow-up.

