In this chapter of Tools and Techniques Clinical, 3D transoesophageal echocardiography for selecting and guiding in percutaneous mitral valve repair using MitraClip® is discussed. The following is a summarised overview of this technique. The complete, unabridged version with images is available online at: http://www.pcronline.com/eurointervention/78th_issue/150
Background
This report provides a brief, illustrative and practical approach for the screening of patients with mitral regurgitation who are candidates for MitraClip® therapy and for procedural guidance using 3D-TOE, providing state-of-the-art practice for imagers (what and how to assess mitral valve with 3D-TOE) and interventionalists (how to interpret and translate 3D-TOE images for the intervention), as summarised in Figure 1.
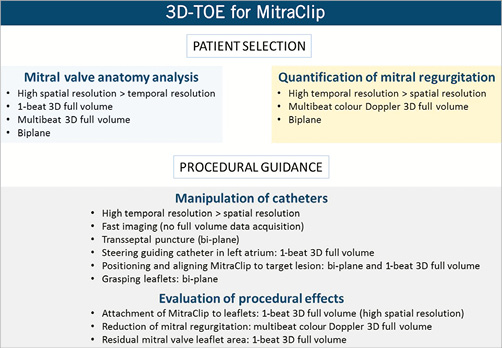
Figure 1. Summary of 3D transoesophageal echocardiography (3D-TOE) for patient selection and procedural guidance for MitraClip® treatment.
Patient selection
The selection of patients who are candidates for MitraClip® therapy includes thorough analysis of the anatomy of the mitral valve and mechanism as well as the severity of mitral regurgitation. Evaluation of the anatomy requires high spatial resolution, whereas accurate quantification of mitral regurgitation relies on high temporal resolution (high frame rate data). Using 3D-TOE, anatomical and functional data can be obtained with three different acquisition modes: 1-beat full volume, multibeat full volume, biplane view.
3D colour Doppler data can be obtained with any of these acquisition methods. However, high temporal resolution must prevail over spatial resolution and therefore colour Doppler 3D multibeat full volume imaging and the biplane view will be the acquisition modes of choice.
ANALYSIS OF MITRAL ANATOMY
The mitral valve en face or “surgical view” comprises 3D visualisation of the entire mitral valve as seen from the left atrium, including mitral annulus, all anterior (A1-A2-A3) and posterior (P1-P2-P3) leaflet scallops, as well as both anterolateral and posterolateral commissures. The mitral valve can also be analysed using the biplane view, setting the bicommissural view as reference, mostly found at about 60º, and bisecting each level of the mitral valve (from anterolateral P1 scallop over central A2 scallop to posteromedial P3 scallop) to display the simultaneous left ventricular outflow tract view with the anterior and posterior scallops of the mitral valve at each level.
Adherence to initial EVEREST II criteria for technical feasibility yields high procedural success rates. In patients with organic mitral regurgitation, the exact location of the lesions (prolapse/flail) and numbers of leaflet scallops involved should be assessed (Figure 2). A flail width ≤15 mm and a flail gap ≤10 mm are recommended. In patients with functional mitral regurgitation, a coaptation length ≥2 mm should be present at the level of the mitral regurgitant jet origin and the coaptation height should be ≤11 mm.
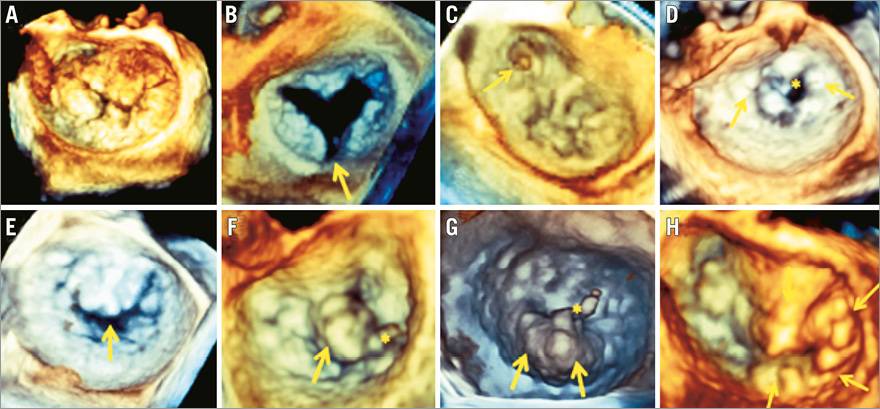
Figure 2. Additional value of 3D mitral valve echocardiography for anatomic and morphologic assessment in screening for MitraClip® therapy. All patients in the examples shown presented with severe mitral regurgitation but were anatomically not eligible for MitraClip® therapy. A) Complex Barlow degeneration involving prolapse of all mitral segments. B) Posterior leaflet cleft. C) Anterolateral commissural prolapse. D) Severe rheumatic stenosis (*) with diffuse calcifications and commissural fusion (arrows). E) Loss of central coaptation during systole. F) A2 flail with chordal rupture (*), flail width 17 mm. G) P2 flail with chordal rupture (*), flail width 22 mm. H) Complex Barlow with prolapse of A2, A3, posteromedial commissure and P3 scallops.
MitraClip® (Abbott Vascular, Santa Clara, CA, USA) therapy reduces mitral valve area by about 25 to 50% and therefore the mitral valve area should be ≥4.0 cm² to avoid development of significant leaflet stenosis. Mitral valve area can be assessed on 3D planimetry at multiplane reformation analysis at the maximal opening of the tip of the leaflets during systole.
QUANTIFICATION OF MITRAL REGURGITATION
Quantification of the effective regurgitant orifice area (EROA), most often by the proximal isovelocity surface area method (PISA), is the gold standard for quantifying regurgitation severity as it depends less on haemodynamic loading conditions. An EROA of ≥40 mm² and ≥20 mm² defines severe mitral regurgitation for organic and functional mitral regurgitation, respectively.
The origin, number and exact location of the regurgitant jet(s) are most easily evaluated on colour Doppler 3D-TOE, applying the en face view as well as the biplane mitral valve view.
Procedural guidance
Comprehensive guiding of a MitraClip® implantation procedure requires extensive use and switching among different 3D-TOE modalities (Figure 3); fluoroscopy guiding is limited since the non-calcified mitral valves are not well depicted. One-beat 3D full volume and biplane acquisitions are the most common 3D modes of visualisation. Procedural results in terms of reduction of mitral regurgitation severity and clip attachment require high temporal and spatial resolution, respectively.
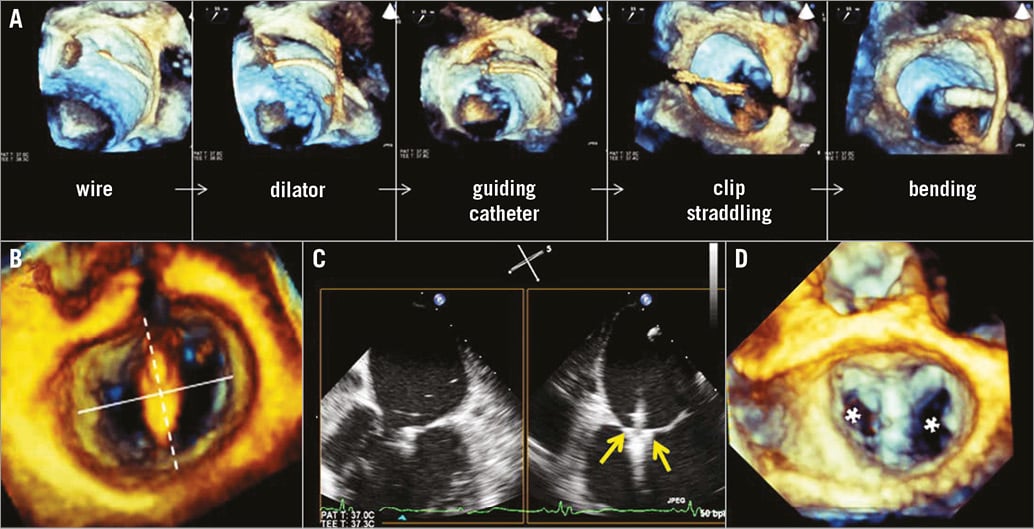
Figure 3. 3D-TOE guidance of MitraClip implantation. A) Large 1-beat 3D full volume views of interatrial septum and mitral valve showing consecutive steps of introducing the guiding catheter into the left atrium and bending towards the mitral valve. This anatomic overview is used for orientation and to make sure no anatomic structures are damaged during manipulation. B) Perpendicularity to the line of coaptation is found when, on the en face mitral view, the clip points towards the centre of the aortic valve. C) Biplane view of leaflet grasping: the clip is closed (arrows), approximating both the posterior and anterior mitral valve leaflets. D) One-beat 3D full volume of double orifice (asterisks) mitral valve, indicating perpendicular clip alignment.
A full description of manipulation of catheters can be found online.
EVALUATION OF PROCEDURAL EFFECTS
MITRAL REGURGITATION REDUCTION
Firstly, reduction of mitral regurgitation should be evaluated. A double orifice mitral valve is created by MitraClip® therapy, often splitting the original jet into two small residual jets. Acute procedural success is defined as reduction of mitral regurgitation to ≤grade 2.
LEAFLET INSERTION
Secondly, to avoid the risk of future clip detachment, it is essential to ensure that both leaflets are adequately captured and inserted between the grippers and both clip arms. The leaflet length inserted into the clip arms (ideally about 5 mm) can be assessed by subtracting the remaining mobile leaflet length from the preprocedural mobile leaflet length. At the bicommissural mitral valve level, the clip should be visualised transecting the mid portion by the leaflets. On the short-axis transgastric view the double orifice can be appreciated, similar to a 3D view, resembling a figure of eight or dog-bone pattern. Additionally, the 1-beat 3D full volume view is essential to confirm perpendicular alignment of the clip arms to the mitral coaptation line.
MITRAL VALVE AREA REDUCTION
Finally, transmitral gradient and mitral valve area are evaluated to ascertain no functional mitral stenosis is created by the MitraClip® intervention.
Conclusions
3D-TOE is very helpful for optimal candidate selection and procedural guidance in patients treated with MitraClip® therapy. Superior anatomic performance and comprehensive evaluation of regurgitant jet characteristics represent the cornerstones for eligibility assessment. Standardised mitral valve display, alternating 1-beat 3D full volume and biplane acquisitions, improve procedural guidance.
Conflict of interest statement
P. Debonnaire is a Crossroads Faculty member and listed as imaging proctor for MitraClip (both Abbott Vascular). He receives speaker fees from Philips Healthcare and Abbott Vascular. V. Delgado has received consulting fees from St. Jude Medical and Medtronic. The Department of Cardiology of Leiden University Medical Center has received grants from GE Healthcare, Lantheus Medical Imaging, St. Jude Medical, Medtronic, Boston Scientific, Biotronik, and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

