Introduction
Patients with severe functional and/or organic mitral regurgitation (MR) deemed unfit for traditional surgical mitral valve (MV) repair or MV replacement may be eligible for percutaneous edge-to-edge repair with the MitraClip® system (Abbott Vascular Structural Heart, Menlo Park, CA, USA)1. Procedural challenges such as optimal clip location placement, and the ideal number of clips to be placed are met using trial and error and are susceptible to procedural inefficacy resulting in residual MR or mitral stenosis.
In this study we present a novel computer-based imaging software (FEops, Ghent, Belgium), which allows the creation of a patient-specific three-dimensional reconstruction of the MV. Using preprocedural and post-procedural transoesophageal echocardiography (TOE) images of two patients, we demonstrate an accurate model, which shows valve anatomy across the cardiac cycle, including mitral valve regurgitation. Using this software, future MitraClip procedures may be preceded by a virtual analysis of the MV, which will allow a careful assessment of the feasibility of repair, ideal clip location, and number of clips to be placed.
Methods
A three-dimensional (3D) model of the mitral valve was produced based on 3D TOE (Figure 1A). TOE images of the two patients were acquired during the procedure. In future prospective studies, the images will be collected well in advance of the MitraClip procedure, allowing image analysis and thus preprocedural planning (the duration of MV modelling takes one to four hours, depending on imaging quality). Image acquisition requires three to four heartbeat interpolation under ventilator breath hold (if under general anaesthesia) with a target frame rate of 20-25 frames per second and full visualisation of the annulus and leaflets. Images are exported using QLAB (Philips Healthcare, Best, the Netherlands) and the four-dimensional (3D + time) DICOM stack of images is imported into 3D Slicer, a platform for medical image analysis and visualisation2. Manual segmentation (red and blue dots) of the annulus (Figure 1B) and both valve leaflets (Figure 1C) was performed in the mid-diastolic frame.
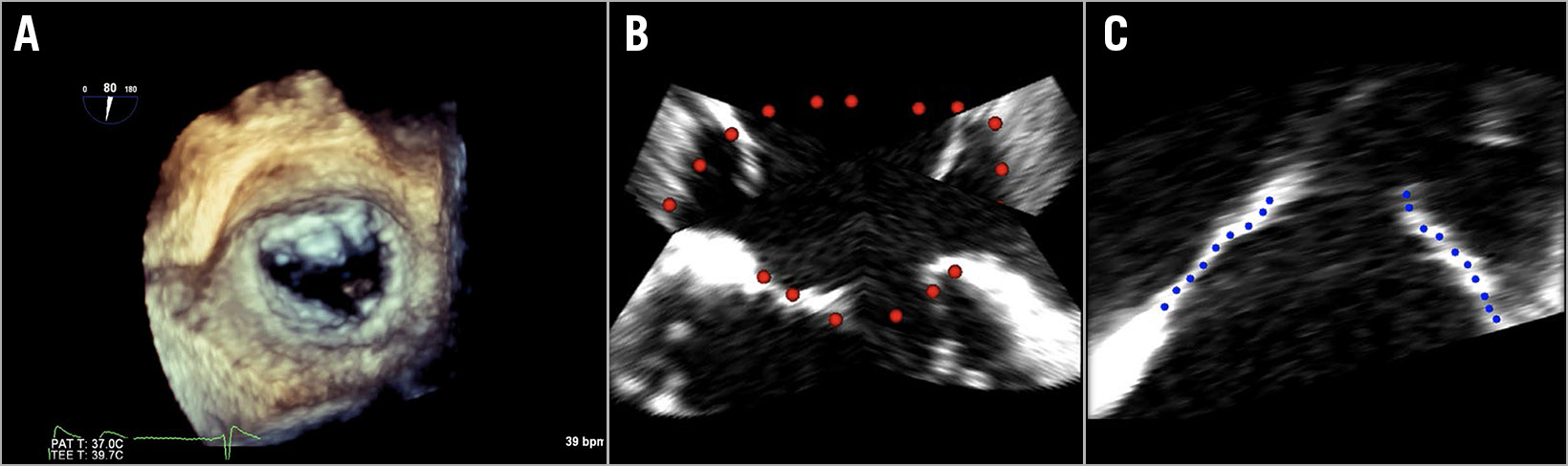
Figure 1. TOE image tracing. A) Three-dimensional TOE image of the mitral valve in surgical view. Manual segmentation of annulus (B) (red dotted line) and valve leaflets (C) (blue dotted line).
Next, a template mesh registration of the leaflet point cloud was performed (Figure 2A), which was subsequently used to add chordae virtually (Figure 2B). Annular displacement boundary conditions (Figure 2C) were defined based on the true annulus position in diastole (blue) and systole (red). The finite model (Figure 2D) is shown in surgical view; only the leaflets are shown.
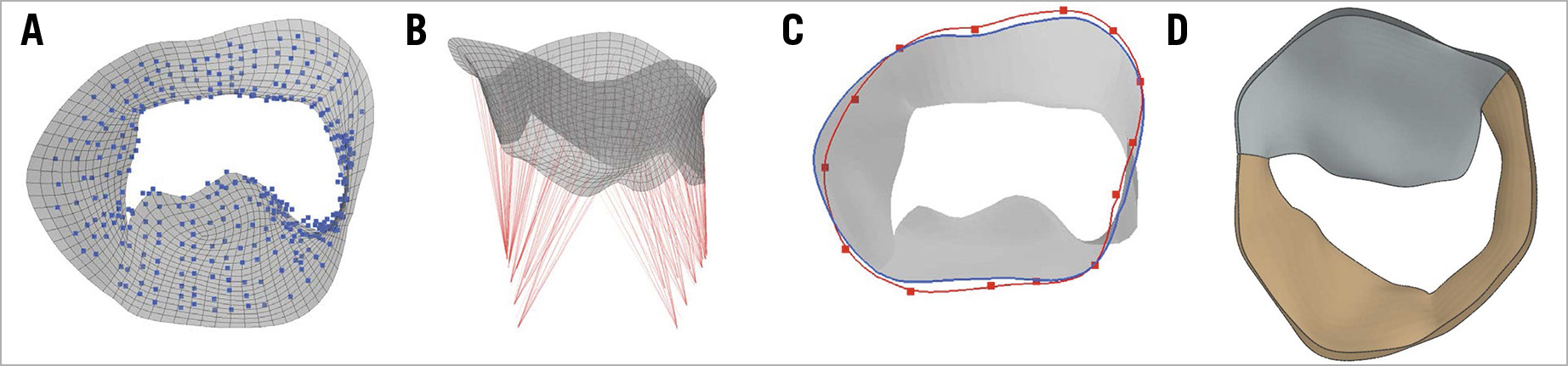
Figure 2. Simulation pre-processing. A) Template mesh registration of the leaflet point cloud. B) Virtual addition of chordae. C) Determination of annular displacement boundary conditions, based on true annulus position in diastole (blue) and systole (red). D) Finite model shown in surgical view with anterior (grey) and posterior (brown) leaflets.
Results
Two patients with grade 4 MR were analysed. Mid-diastolic models for both patients are shown on the left in Figure 3. Systolic closure (right) of the mitral valve was simulated under a pressure load (120 mmHg, or 0.016 MPa).
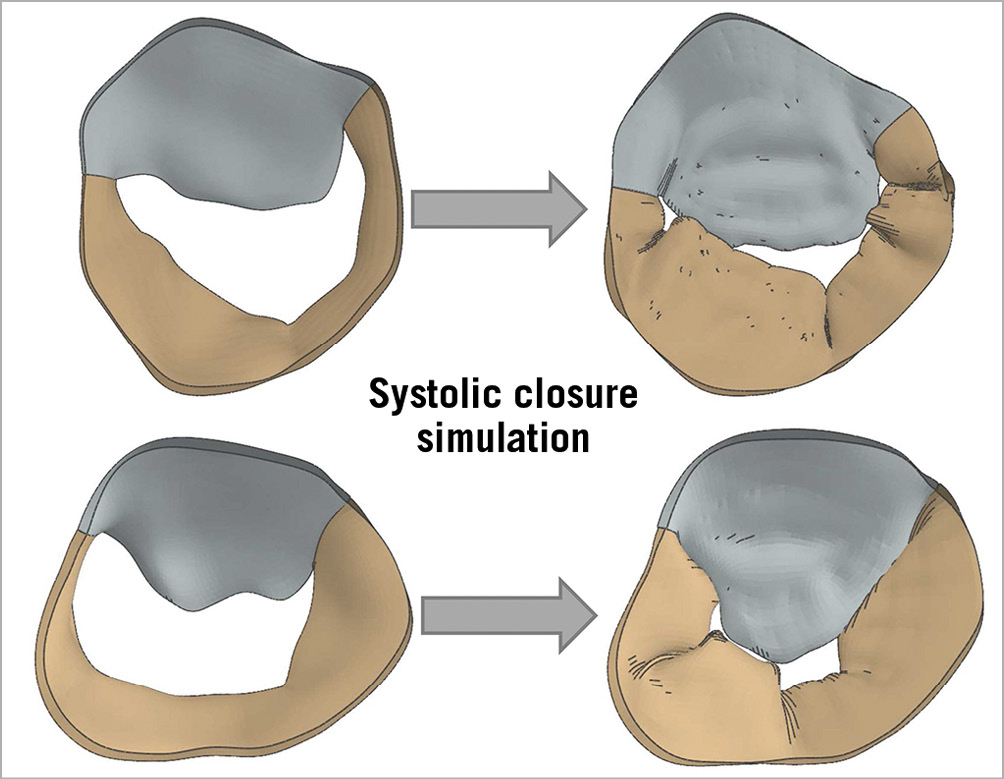
Figure 3. Systolic closure simulation. Mid-diastolic models for both patients (left). Systolic closure (right) of the mitral valve was simulated under a pressure load. In both patients, retraction of the posterior valve leaflet can be seen with resulting mitral regurgitation.
The simulated leaflet position (purple lines) derived from the systolic closure model of both patients (top and bottom) was compared with true TOE imaging (Figure 4A). In Figure 4B, a colour plot is displayed showing the distance in millimetres between the simulated systolic mitral valve in and the true systolic mitral valve contours traced from TOE images. The average distance error for patient 1 (left) was 0.9 mm, while the average error for patient 2 (right) was 0.7 mm.
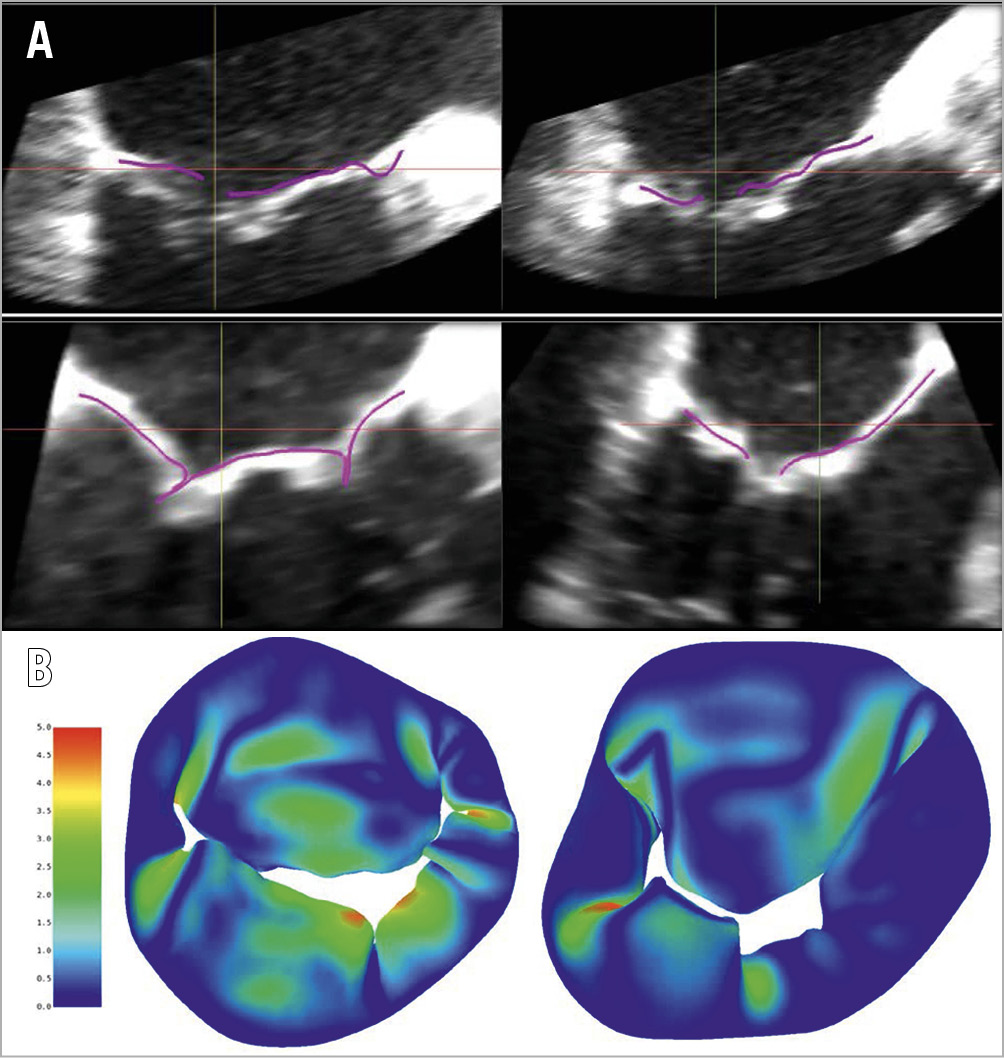
Figure 4. Qualitative and quantitative comparison with true echocardiographic imaging. A) Simulated leaflet position (purple lines) derived from the systolic closure model of both patients (top and bottom), compared with true TOE imaging. B) Colour plot showing distance (millimetres) between simulated systolic mitral valve and true systolic mitral valve contours traced from TOE images.
A MitraClip was virtually added to the model (Figure 5A). MitraClip location was estimated based on the true post-clip 3D TOE images. In both patients (top and bottom), systolic closure with the MitraClip (two clips in patient 1, top) was simulated (Figure 5B).
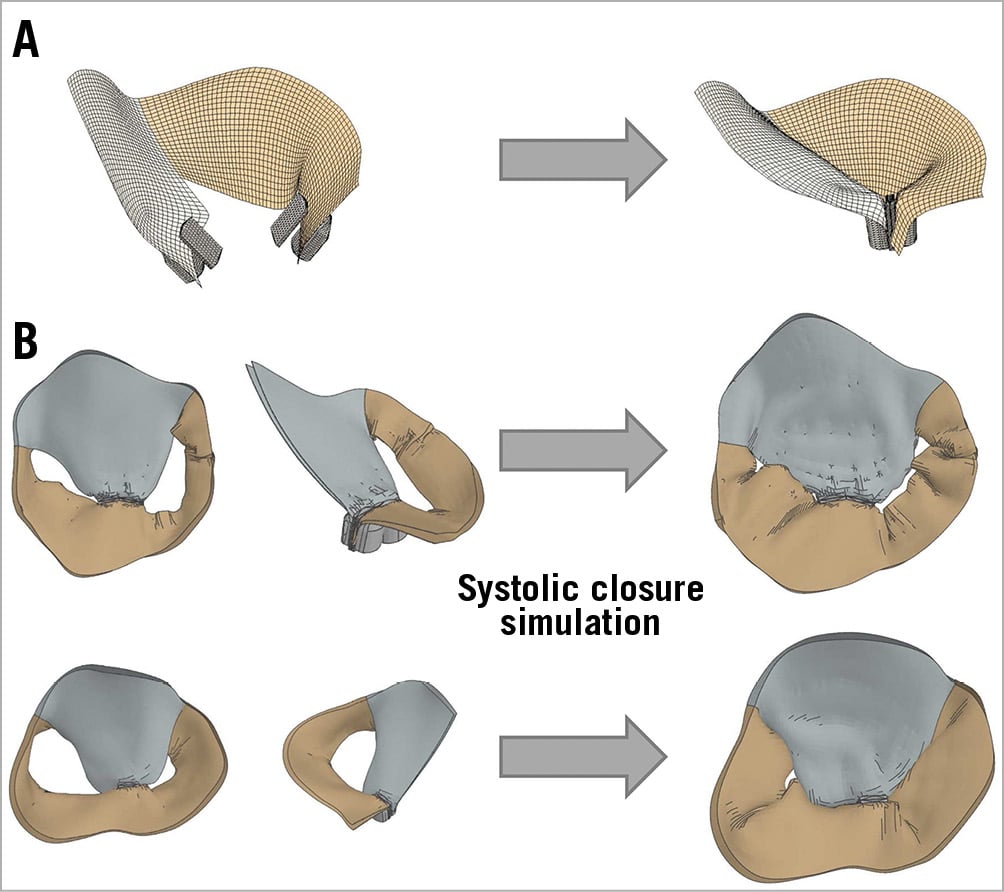
Figure 5. MitraClip simulation. A) Virtual MitraClip addition. B) Systolic closure simulation.
Patient 1 (Figure 5, top) shows a small residual MR at A3-P3, which coincides with a grade 1 residual MR in the true position (Figure 6A). Likewise, patient 2 (Figure 5, bottom) shows a negligible commissural residual MR, which coincides with the insignificant MR in the true position (Figure 6B).
Figure 6 shows TOE colour Doppler images of residual MR of both patients. Patient 1 (Figure 6A) shows a grade 1 residual MR at A3-P3. Patient 2 (Figure 6B) shows an insignificant residual MR.
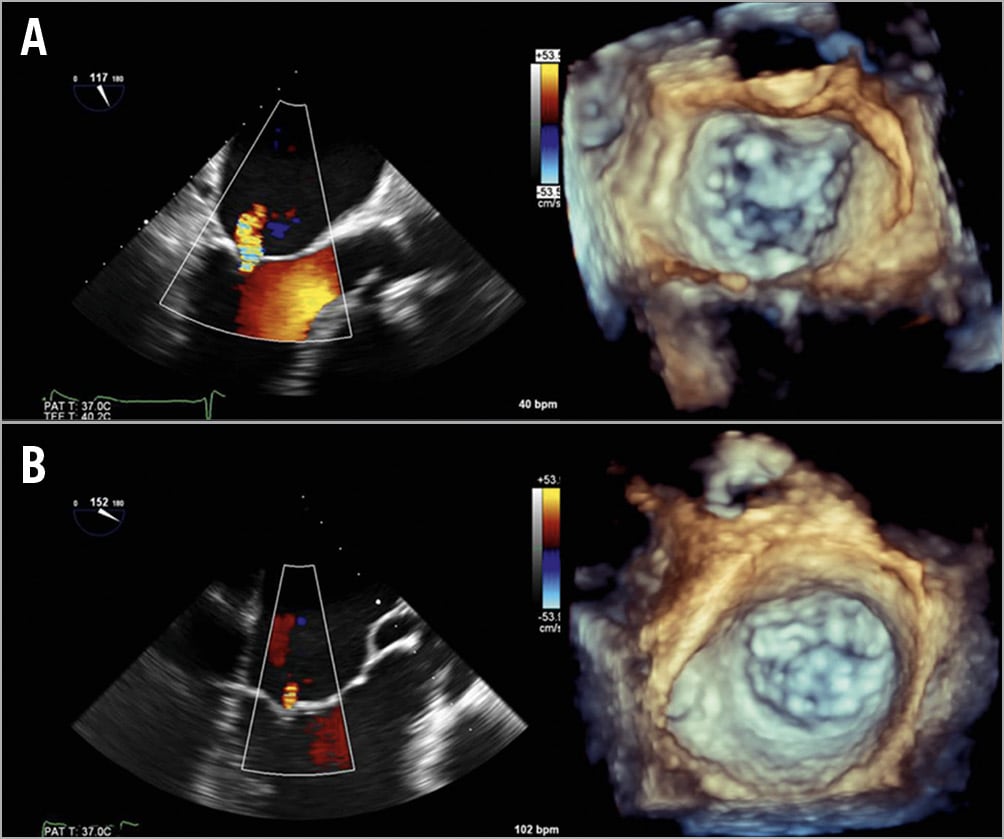
Figure 6. True residual MR. Two-dimensional TOE colour Doppler images of residual mitral regurgitation of patient 1 (A) and patient 2 (B), with corresponding 3D TOE in systole.
Discussion
In this study, proof-of-concept of a computer-based simulation of the MV, including MitraClip, was demonstrated. Preprocedural and post-procedural TOE images were used to create a model retrospectively, which can be seen as a representation of true conditions. A high level of agreement was observed between the computer-simulated model and the true leaflet positions, as seen in the colour plot. Fine-tuning of the technical aspects with more patient data is likely to reduce average error. Qualitative assessment of the location of the residual MR coincided well with the true residual MR, as seen in the colour Doppler images.
Preprocedural virtual MitraClip placement may be of benefit for several reasons. Firstly, the mechanism of the MR may be visualised more easily in a 3D fashion. Secondly, the feasibility of clip placement may be assessed by analysing valve movement/length and coaptation area. Finally, an estimation of clip(s) location can be made with subsequent residual MR, residual mitral valve area and pressure gradient, a significant step forward compared with the trial and error aspect in the actual procedure.
Limitations
The technique outlined in this article is susceptible to limitations. Simulation is dependent on TOE image quality and image acquisition should be performed by an experienced operator. Furthermore, segmentation of the annulus and valve leaflets is performed manually, leaving software simulation subject to human error. Fortunately, as experience grows with time, so will accuracy.
Conclusion
The method described represents an innovative technique where an accurate representation of the mitral valve is created using 3D TOE. In the future, using this software, virtual simulation of the mitral valve and subsequent MitraClip placement may be performed prior to the actual procedure. Feasibility, and the ideal number of clips to be placed and their ideal location may be determined beforehand, which may improve procedural planning, result rates, safety, and cost-effectiveness. Although the validity of the model has to be tested in future prospective studies, this study represents the first step in the new landscape of software-based treatment planning.
|
Impact on daily practice Virtual placement of one or more MitraClips may predict residual mitral regurgitation and may therefore increase success rates, safety, and cost-effectiveness. In the clinical setting, the operator will be able to pinpoint the exact location of clip placement and the number of clips to be placed. Likewise, patients who may not benefit from MitraClip placement may be identified. |
Funding
This study was funded by Abbott Vascular Netherlands, Heerlen, the Netherlands. Abbott was not involved in the collection, analysis, or interpretation of the data, nor were they involved in the preparation, review, or approval of the manuscript.
Conflict of interest statement
M. De Beule is the CEO of FEops. S. De Bock is an employee at FEops. The other authors have no conflicts of interest to declare.

