Introduction
The native heart valves and, in particular, the aortic valve have a complex geometry that provides both ideal opening and closing geometries through an anatomic joining of a tubular inflow structure of the left ventricular outflow tract and an expansion of the valve sinuses above the hinging point of the valve leaflets defined by the aortic valve annular ring (Online Figure 1).
A central challenge to the formation of a transcatheter heart valve (THV) is the confinement of the operating leaflets within a partially sealed tubular structure while preserving effective opening and closing valve behaviour without the benefit of the natural mechanism of the sinuses of Valsalva in a single valve and leaflet geometry. The compromises reflected in the available THV designs have produced THVs that require large delivery catheters which impose technical difficulties and clinical risks on the patient. The Colibri heart valve (CHV) was designed to address these shortcomings.
Description - technical specifications
LEAFLET GEOMETRY
The Colibri heart valve design answers these challenges by employing conical rather than spherical cusp geometry, thereby reproducing some benefits of the latter with near-elliptical leaflet cross-section that progressively decreases moving proximal to the plane of leaflet apposition while being readily conformed on outward radial compression in the valve opening phase into a substantially flat folded construct (as in origami) against the interior tubular walls of the containing frame (Figure 1). This favourable resolution of the conical geometry in the opening phase expresses the opening efficiency of this valve design with a large effective orifice area and low transvalvular energy losses. Further, the conical cusps are particularly suited for compression and containment within a collapsible frame for transcatheter delivery.
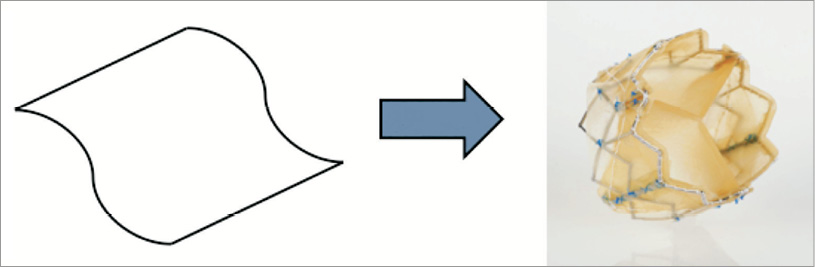
Figure 1. Colibri TAVI valve with folded membrane construction. Continuous surfaces with leaflet integral to cusp. Only ~150 sutures used for mounting.
Each integral cusp and leaflet structure is attached to the frame and simultaneously closed along a seam that is non-load-bearing. The sutures are principally directed to mounting of the cusps and therefore number far fewer than in extant THV designs, with current production models utilising only approximately 150 sutures. In addition, the reduction in the number of sutures reduces the material mass within the frame envelope favouring the compression and low profile of the mounted device.
LEAFLET MATERIAL
Recognising the need to reduce leaflet mass, a first design decision was to reduce leaflet thickness. Through innovative tissue processes, the thickness of the porcine pericardium used was reduced to roughly one third of that in competing designs, while retaining the structural protein content. Next, recognising that about 70% of the mass of tissue is water, the valve was designed to allow for gradual dehydration in the mounting process, further enabling pre-mounting the valve in manufacture, with the tissue structures in total occupying approximately 10% of the original volume. The combined efficiencies of the material engineering, flat folded geometry, and minimised suture content finally enabled the Colibri dry, pre-mounted, pre-packaged, ready-for-use TAVI valve construct to be delivered through a 14 Fr introducer sheath.
DRY TISSUE PRE-MOUNTING REQUIRES STRESS-TOLERANT TISSUE
In order to achieve a dry, thin crimped tissue that confers a lower mass and profile, high tensile strength is required. Crimping and mounting in the dry state impose high stresses upon the tissue material which require that the tissue material should possess equally high tolerance of these stresses. Typical valve leaflet tissue materials have ultimate tensile strength, or breaking stress values in the 6-8 megaPascal (mPa) range with 12 mPa representing a very high stress performance. In response to this design requirement, Colibri has developed tissue materials with ultimate tensile strengths in the 30-50 mPa range, as shown in Online Figure 2. It should be emphasised that this performance is not required for the operation of the valve in vivo (requiring only 10’s of kiloPascals), but rather for the tolerance of forces imposed by the crimping and mounting in the dehydrated state.
In addition to the dry valve having lower mass and therefore being able to be pre-mounted upon the delivery balloon catheter and encapsulated within the delivery sheath, it can also be packaged and sterilised in the dry state. This simplifies shelf storage and eliminates storage in glutaraldehyde solution. Therefore its handling is entirely analogous to that of the pre-mounted coronary stent devices.
THE PRE-MOUNTED PRE-PACKAGED COLIBRI HEART VALVE
Following the general design principles as outlined above, the Colibri heart valve has been created as a successful embodiment of a dry, pre-mounted, pre-packaged TAVI valve that is encapsulated upon a delivery balloon catheter within a 14 Fr introducer sheath and packaged integrated upon the delivery catheter ready to use (Figure 2 and Figure 3). The Colibri system is simply removed from the package (Online Figure 3), the ports are flushed and the system is introduced into the body. Though dry on insertion, the leaflets begin hydrating and operate immediately upon deployment.
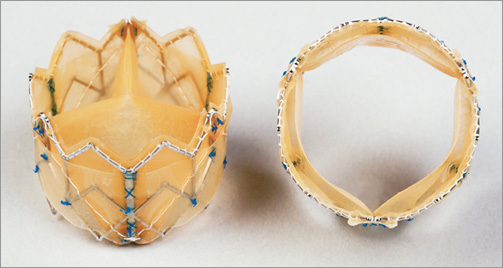
Figure 2. The Colibri heart valve TAVI device.

Figure 3. The Colibri valve pre-mounted and encapsulated within the delivery system (shown unsheathed).
Implantation procedure
FIRST-IN-HUMAN CASE
The 24 mm-sized Colibri TAVI valve is pre-mounted and compressed upon its balloon-in-balloon delivery catheter at manufacture and is packaged ensheathed within a 14 Fr introducer, sterilised and ready for insertion into the patient. We present the results and early clinical follow-up of the first human implantation of this Colibri heart valve in an initial feasibility study.
An elderly, frail, hypertensive, bedbound female patient with minimal effort dyspnoea and recurrent syncope (NYHA Class IV) was referred for treatment. Echo-Doppler showed severe aortic stenosis with mean gradient 89 mmHg, aortic valve area (AVA) 0.7 cm2 and moderate aortic insufficiency. The left ventricular ejection fraction was 60%. The extent of coronary artery disease was not critical. The aortic cusps were severely calcified (Figure 4). Surgical valve replacement was not available to her. The minimum diameter of the femoral arteries was approximately 5.0 mm, precluding the transfemoral approach with available TAVI systems. The pre-procedural characteristics of the patient are shown in Table 1 and Table 2.
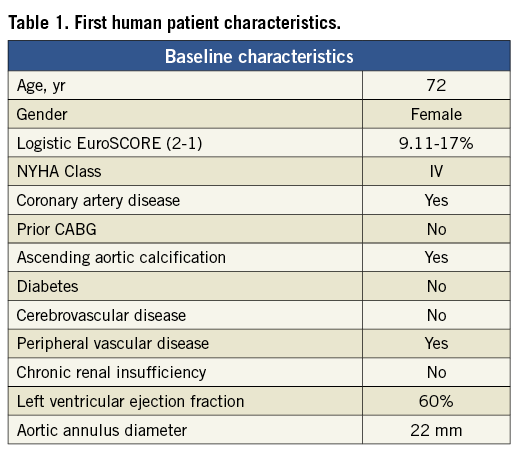
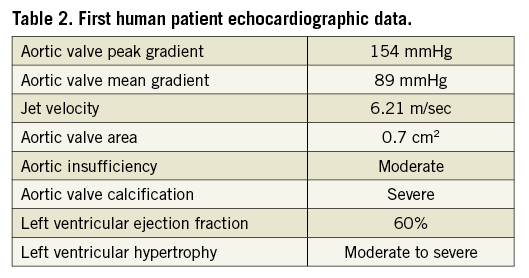
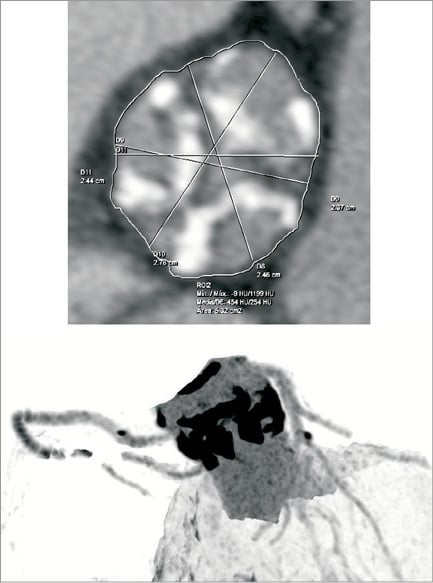
Figure 4. First human patient aortic valve with severe calcification.
On November 29, 2012, the procedure was conducted at the Cardiovascular Department CEDIMAT, Centro de Diagnóstico y Medicina Avanzada y Telemedicina, Dominican Republic, by a team led by Dr Pedro Ureña. Under general anaesthesia, the patient underwent implantation of the Colibri heart valve in the catheterisation laboratory under fluoroscopic and transoesophageal echo guidance. Out of concern for the small-calibre femoral arteries, the right femoral access site was surgically exposed to ensure entry to the femoral artery. After initial balloon aortic valvuloplasty with an 18 mm balloon, the degree of aortic regurgitation increased. On removal from the package, the CHV system was prepared simply by flushing the lumens and aspirating the balloon lumens. After insertion over the guidewire, the Colibri valve delivery system was advanced out of the 14 Fr sheath in the descending thoracic aorta and then readily traversed to the aortic valve plane. It was then deployed under rapid pacing by sequential inflation of the 12 mm inner balloon, then the 24 mm outer balloon of the balloon-in-balloon catheter. The aortic mean gradient decreased to 12 mmHg and the AVA increased to 2.3 cm2. Mild paravalvular leak (PVL) was identified (Figure 5, Figure 6 and Online Figure 4).
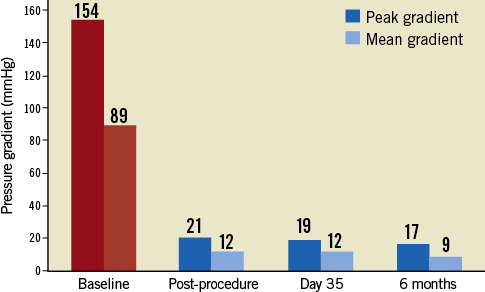
Figure 5. Transvalvular pressure gradient at follow-up.
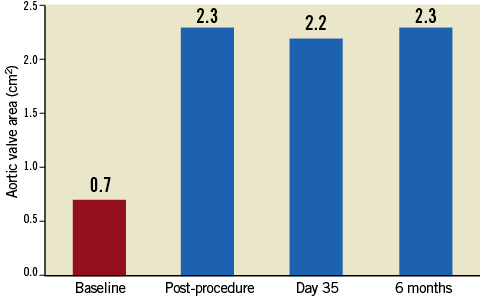
Figure 6. Aortic valve area at follow-up.
Clinical data
Following the CHV implantation the patient experienced prompt symptomatic relief. A follow-up echocardiogram performed at seven days showed trace PVL and transvalvular mean gradient of 12 mmHg. The echocardiogram at 30 days showed no aortic insufficiency (AI) or PVL, the same mean gradient of 12 mmHg and AVA of 2.2 cm2. At six months of clinical follow-up the patient was fully ambulant with NYHA Class 1 functional capacity and preserved AVA of 2.3 cm2 with mean gradient of 9 mmHg (Figure 5, Figure 6 and Online Figure 4). The results of transoesophageal echocardiography at 160 days are shown in Figure 7.
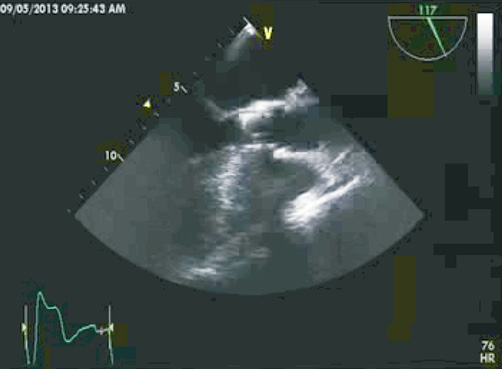
Figure 7. Transoesophageal echocardiogram of Colibri TAVI implant at 6-month follow-up (Moving image 1).
In the first human case of this type, clinical outcome six months after implantation showed maintained AVA and sustained benefits.
Discussion
The design and execution of the Colibri heart valve from first principles to a successful embodiment of a dry pre-mounted, pre-packaged, ready-for-use, low-profile TAVI valve illustrate the practical benefits of a careful analysis of design requirements and assumptions and a focus upon adapting to challenges posed by the resulting design requirements.
The many benefits of the Colibri technology have the potential to advance the field of transcatheter valve therapy in important ways. The pre-mounted dry valve is ready to use off the shelf from the package into the patient. The valve itself does not require preparation and mounting, and therefore eliminates any potential associated errors of misalignment or inadequate crimping. The simplified preparation of the system permits rapid preparation, insertion and deployment of the CHV in the event of haemodynamic crisis or in a valve-in-valve scenario. Further, preparation does not require company personnel, and simplified training of operators can be conducted at centralised locations.
The low profile expands the application of the transfemoral approach to patients with small femoral arteries, including many women and those of certain ethnic groups, such that the vast majority of patients may be expected to be candidates for it. As such, the resource burdens of the transapical approach may be reduced. The low profile may also reduce the rate of vascular access-site complications which have been associated with bleeding events and mortality. The reduced bulk and stiffness of the delivery system also have the potential to reduce the forces acting on the aorta in retrograde passage, which in turn may reduce the potential for injury, such as embolic stroke.
Finally, the dry, pre-mounted, ready-to-use Colibri system is well suited for dissemination of TAVI therapy to resource-poor facilities everywhere.
Conclusion
Transfemoral implantation of the Colibri pre-mounted and pre-packaged transcatheter aortic valve in its 14 Fr delivery sheath is feasible and efficient. In the first human case of this type, clinical outcome six months after implantation showed sustained benefits. This case suggests that the Colibri dry, low-profile, ready-to-use TAVI technology can provide procedural efficiencies and enable the transfemoral treatment of patients with small femoral arteries. Further, the preserved AVA of 2.3 is atypically high for TAVI devices and suggests that the valve design may minimise energy losses. Early follow-up results of this first patient implantation are thus far consistent with the expected benefits observed in the known TAVI experience.
Conflict of interest statement
R.D. Fish and D. Paniagua are board members and major shareholders of the company, Colibri Heart Valve, LLC. P. Urena is an unpaid consultant to the company, CHV, LLC. B. Chevalier serves on the advisory board of the company (CHV, LLC) and holds a minor shareholder interest.
Online data supplement
Moving image 1. Transoesophageal echocardiogram of Colibri TAVI implant at 6-month follow-up.
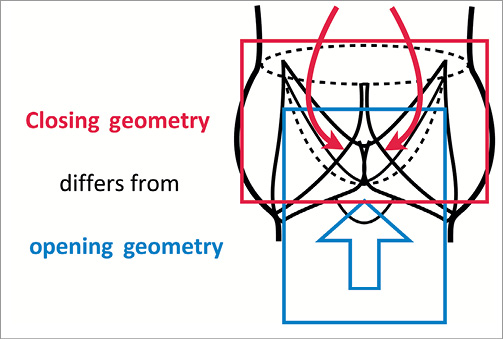
Online Figure 1. Aortic valve closing geometry compared to opening geometry.
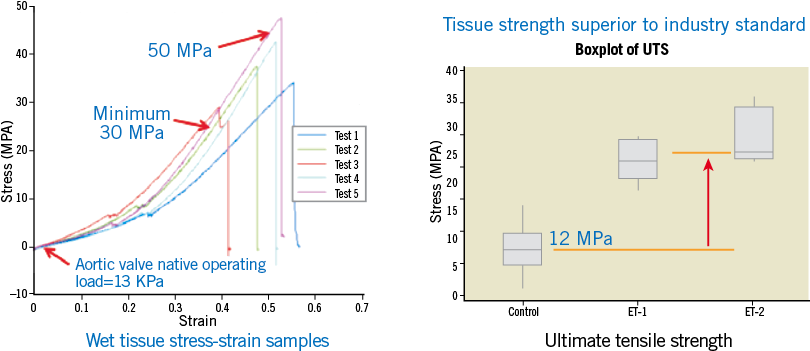
Online Figure 2. High tensile strength tissue membrane enables crimping and mounting at manufacture.

Online Figure 3. The Colibri pre-mounted, pre-packaged TAVI valve in its delivery system is ready to use from the package.
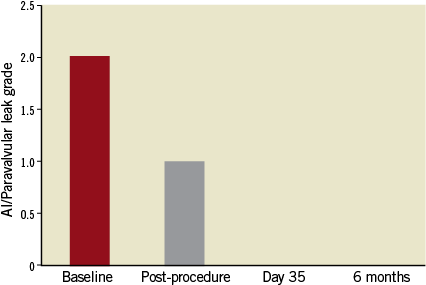
Online Figure 4. Aortic insufficiency or paravalvular leak at follow-up.

