
Abstract
Aims: The REPRISE IIE trial aimed to evaluate outcomes following transcatheter aortic valve implantation of the fully repositionable and retrievable LOTUS valve with a unique seal designed to minimise paravalvular leak (PVL).
Methods and results: This prospective, multicentre study enrolled 250 patients with severe aortic stenosis considered high-risk for surgery by a multidisciplinary Heart Team. An independent clinical events committee adjudicated events per Valve Academic Research Consortium criteria. Mean age was 84 years; 77% were in NYHA Class III/IV. LOTUS valve implantation produced significant haemodynamic improvements at one year without valve embolisation, ectopic valve deployment, or additional valve implantation. Primary endpoints were met as the 30-day mortality rate in the extended cohort (4.4%, N=250), and mean valve gradient in the main cohort (11.5±5.2 mmHg, N=120) were below (p<0.001) their predefined performance objectives. At 30 days, disabling stroke was 2.8% and new pacemaker implantation was 28.9% in all patients and 32.0% in pacemaker-naïve patients. By one year, all-cause mortality was 11.6%, disabling stroke was 3.6%, 95% of patients alive were in NYHA Class I/II, and there was no core laboratory-adjudicated moderate/severe PVL.
Conclusions: LOTUS valve implantation produced good valve haemodynamics, minimal PVL, sustained significant improvement in functional status, and good clinical outcomes one year post implant.
Abbreviations
CEC: clinical events committee
ITT: intent-to-treat
NYHA: New York Heart Association
PPM: permanent pacemaker
PVL: paravalvular leak
SF-12: 12-item short form health survey
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
TTE: transthoracic echocardiography
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation (TAVI) has become a well-established treatment for symptomatic severe aortic stenosis in poor surgical candidates, but first-generation valves have faced limitations with optimal positioning and post-procedure paravalvular leak (PVL). The LOTUS™ valve system (Boston Scientific, Marlborough, MA, USA) incorporates several features intended to address these challenges. The valve is repositionable and fully retrievable with a unique adaptive seal designed to minimise PVL. In the 120-patient main cohort of the CE-mark REPRISE II trial, all-cause mortality was 4.2% at 30 days1 and 10.9% at one year2. By independent core lab analysis, one patient had moderate and none had severe PVL at 30 days; 88.6% had none/trivial PVL at one year. To gain additional safety/effectiveness data, the study was expanded to enrol 130 more high-risk patients under the same protocol. We describe 30-day and one-year outcomes in the combined 250-patient REPRISE II trial with extended cohort (REPRISE IIE), which comprises the largest one-year LOTUS valve data set published to date.
Methods
The REPRISE II study design and methods have been described previously1 and are summarised below.
STUDY DESIGN
Patients were confirmed eligible by an independent case review committee. The primary device performance endpoint was 30-day mean aortic valve pressure gradient in the main cohort (N=120), as assessed by an independent core laboratory, and was compared to a pre-specified performance goal of 18 mmHg. The primary safety endpoint, 30-day all-cause mortality, was assessed in the full cohort (N=250) and compared to a pre-specified performance goal of 16%. Additional endpoint definitions, pre-specified follow-up measurements based on Valve Academic Research Consortium (VARC) metrics3, and core laboratories have been published previously1. The study complied with the principles of the Declaration of Helsinki and all local and country-specific regulations. Ethics committees approved the study; all patients (or guardians) provided informed written consent before any study-specific tests or procedures. The study is registered at ClinicalTrials.gov (identifier NCT01627691).
STUDY DEVICE
The LOTUS Aortic Valve Replacement System (Boston Scientific) includes a braided nitinol wire frame with three bovine pericardial leaflets premounted on a preshaped delivery catheter and deployed via controlled mechanical expansion. The valve may be repositioned or fully retrieved at any point prior to uncoupling and release. A polymer membrane (Adaptive Seal™; Boston Scientific) surrounds the lower half of the valve and is designed to minimise PVL. Two valve sizes, 23 mm and 27 mm, were available in this study.
STUDY PROCEDURES
Detailed procedural descriptions, including retrieval to change a valve size, have been published previously1,4. All implantations were performed via retrograde delivery through the femoral artery; balloon valvuloplasty was mandated before LOTUS Valve System insertion. After TAVI, patients were treated with aspirin and a thienopyridine for ≥1 month; daily aspirin was recommended indefinitely thereafter as per local standard of care.
Clinical follow-up after discharge was scheduled at one, three, six, and 12 months and annually from two to five years. Before and after TAVI, neurological assessments were performed by qualified personnel for all patients. Health status was evaluated at baseline, one and six months, and at one, three, and five years using the EQ-5D quality of life and 12-item short form health survey (SF-12) questionnaires5. Independent data assessments were provided by core laboratories. An independent clinical events committee (CEC) including cardiothoracic surgeons, interventional cardiologists, and a neurologist adjudicated adverse events.
STATISTICAL METHODS
The primary device performance endpoint was compared to a predefined performance goal of 18 mmHg, as described previously1. The 30-day mortality rate was compared, using data from the intent-to-treat (ITT) analysis set of the combined trial cohorts (N=250), to a predefined performance goal of 16% (9.8% expected rate plus 6.2% test margin) based on published outcomes with the CoreValve® (Medtronic, Dublin, Ireland) and SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA). The study had 80% power to conclude that the primary safety endpoint was met if the one-sided upper 97.5% confidence bound of the observed 30-day mortality rate was <16% with a one-sample z-test p-value <0.025. Additional statistical methods have been described previously1.
Results
PATIENTS
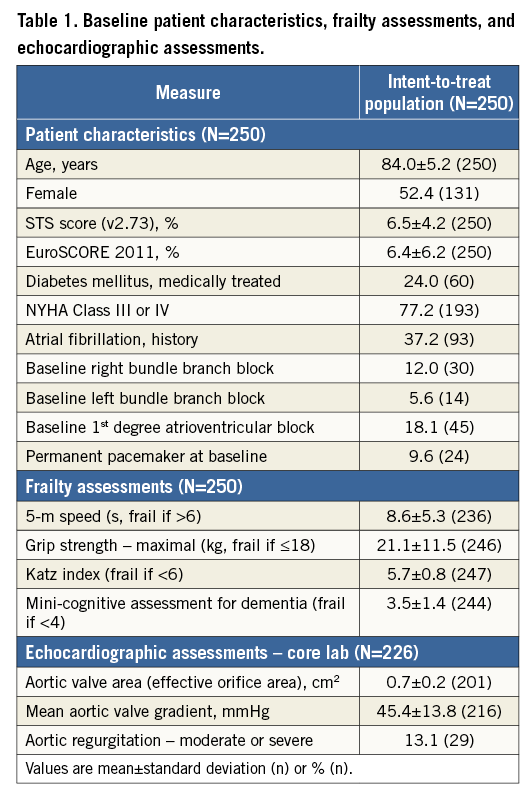
Figure 1 shows patient flow. Two patients did not receive a LOTUS valve during the index procedure because of procedural complications prior to attempted valve implantation. One of them underwent successful LOTUS valve implantation 42 days later during a second TAVI procedure. Thus, the as-treated analysis population included 249 patients. Table 1 shows baseline characteristics. While the mean STS score was 6.5±4.2% (median score: 5.5, range 1.4 to 30.0), 87% of patients had ≥1 and 59% had two frailty measures above threshold.
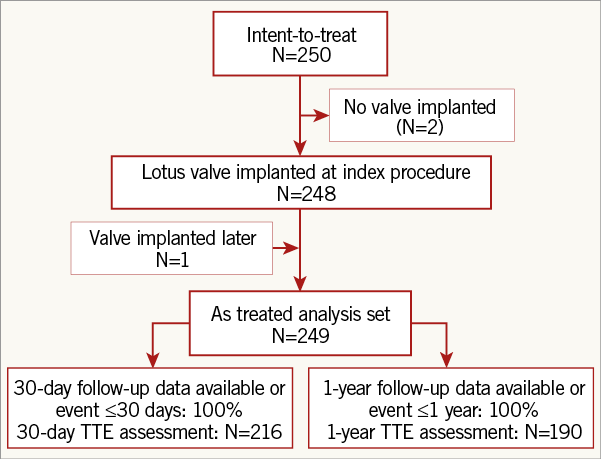
Figure 1. Patient flow to one year. There were 250 patients enrolled between October 2012 and April 2014 at 20 centres in Australia and Europe in the combined REPRISE II main (N=120) and extended cohorts. In two patients, a LOTUS valve was not implanted during the index procedure because of procedural complications; one of these two patients underwent successful implantation of a LOTUS valve during a second TAVI procedure conducted 42 days later. TAVI: transcatheter aortic valve implantation; TTE: transthoracic echocardiography
PROCEDURAL OUTCOMES
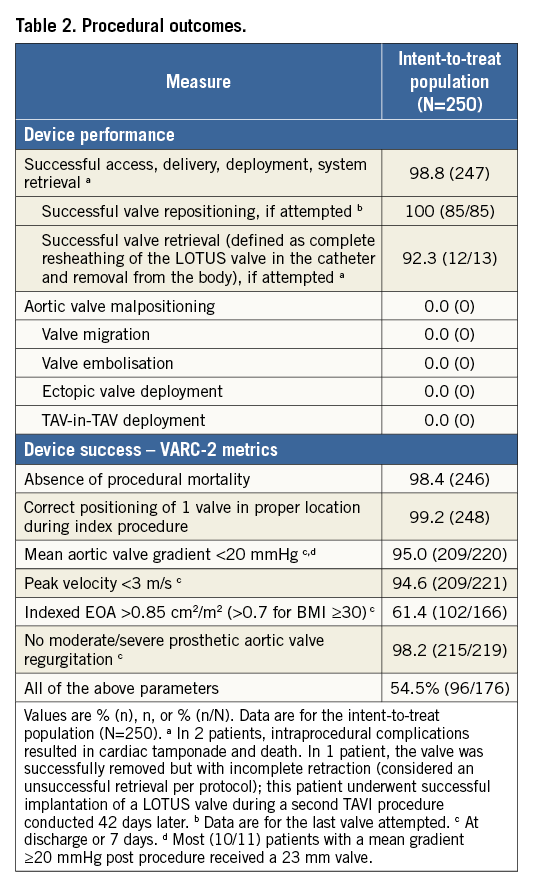
Procedural outcomes are shown in Table 2. Post-dilatation was not recommended and was not performed in any patient. Of 13 patients with valve retrieval, eight (3.2% of 250 enrolled patients) were the result of a change of valve size (all replacement of a 23 mm valve with a 27 mm valve). There was no valve migration, embolisation, ectopic valve deployment, or TAV-in-TAV deployment. The VARC 2 metrics for mean gradient (<20 mmHg), peak velocity <3 m/s), and PVL (no moderate/severe) were each met in ≥95% of patients with evaluable discharge echocardiograms. There were 11 (4.4%) patients who had a mean gradient >20 mmHg at discharge/seven days (Table 2). Of these, six had Doppler velocity indices (DVI) >0.35 suggesting that valve function was normal despite the elevated gradient, four had DVI between 0.25 and 0.35 consistent with mild prosthetic valve dysfunction, and one patient did not have DVI measured. In the four patients with DVI at discharge/seven days between 0.25 and 0.35, all subsequent echocardiograms available to date (including minimum echocardiograms at 30 days, three months, six months and 12 months) showed DVI >0.35 consistent with normal valve function. The one patient who did not have DVI measured at discharge/seven days did not have another echocardiogram performed until the one-year follow-up visit. DVI was 0.3 at one year and 0.56 at two years (the last available echocardiogram to date), suggesting normal valve function at two years. The angiographic core lab reported that there was no significant frame deformation in any of these 11 cases.
PRIMARY ENDPOINTS
The primary device performance endpoint was met, as the 30-day mean aortic valve gradient among main cohort patients was 11.5±5.2 mmHg with a one-sided 98.7% upper confidence bound of 12.6 mmHg, significantly below (p<0.001) the performance goal of 18 mmHg1. In the 250-patient ITT analysis set, all-cause mortality at 30 days was 4.4% with a one-sided 97.5% upper confidence bound of 6.97%, which was below the performance goal of 16.0% (p<0.001). Thus, the primary safety endpoint was met.
VALVE PERFORMANCE AND ADDITIONAL MEASUREMENTS
Valve performance data are shown in Figure 2. Mean values for aortic gradient and effective orifice area, as determined by independent core lab analyses of transthoracic echocardiography (TTE) outcomes, were significantly improved from baseline to discharge and 30 days and remained so at one year (Figure 2A). At 30 days, PVL was absent/trace in 86% of evaluable core lab-adjudicated echocardiograms; there was one case of moderate and no cases of severe leak. These results were sustained at one year as no moderate/severe PVL was observed and 85% of patients had none/trace PVL (Figure 2B).
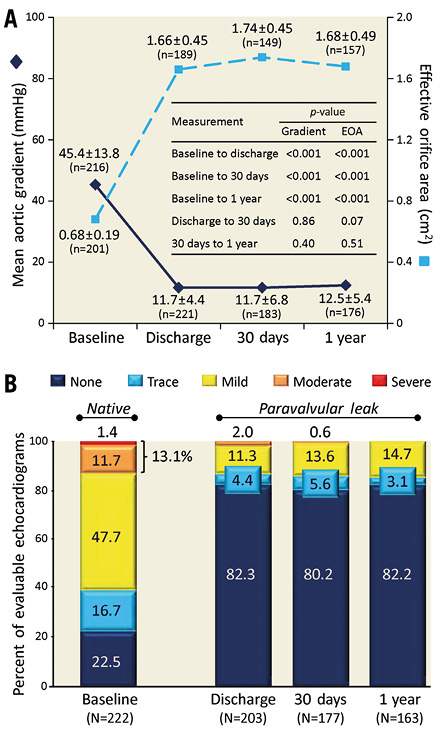
Figure 2. Haemodynamic outcomes and aortic regurgitation. A) Mean aortic gradient and effective orifice area by transthoracic echocardiography. B) Aortic regurgitation by transthoracic echocardiography. Data are for the as-treated population (N=249) and were assessed by an independent core laboratory. Mean±SD. P-values from estimates of the repeated measures and random effects ANOVA model with unstructured covariance.
Progressive improvements over time were seen in NYHA functional class. At baseline, 77% of patients were in Class III/IV, whereas 92% and 95% were in Class I/II at 30 days and one year, respectively (Figure 3). The SF-12 physical health summary score improved from 34.1±9.3 at baseline to 38.1±10.2 at one year, indicating a clinically meaningful enhancement in health-related quality of life (Table 3).
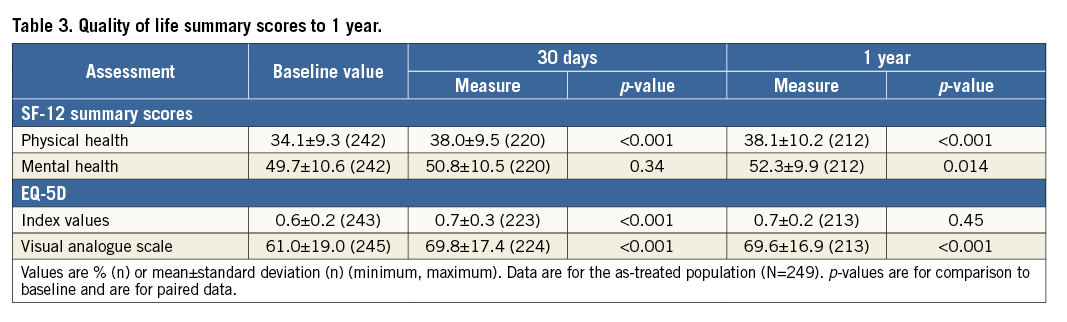
Figure 3. New York Heart Association functional class assessments to one year. Data are for the as-treated population (N=249). There were 10 deaths by 30 days post procedure and 29 deaths in total at one year; p<0.001 for comparison of each time point to baseline.
CLINICAL SAFETY OUTCOMES
Table 4 shows 30-day and one-year VARC-defined safety outcomes in the as-treated population. All-cause mortality was 4.0% and 11.6%, and disabling stroke was 2.8% and 3.6%, respectively. The 30-day stroke rate was similar among patients with or without repositioning or retrieval (p=0.86). Beyond 30 days, two patients were successfully treated for prosthetic valve endocarditis, and three cases of adjudicated valve thrombosis resolved without clinical sequelae with anticoagulation treatment (Table 4).
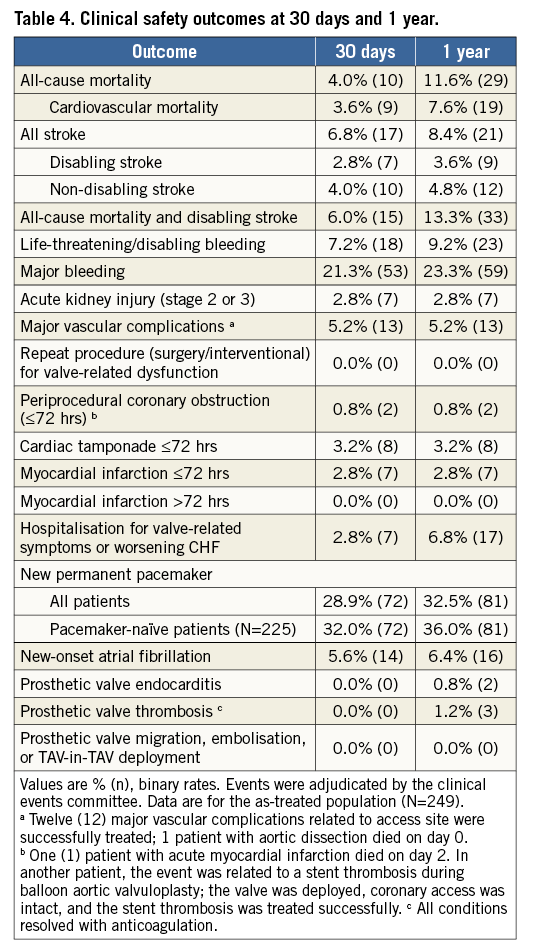
By 30 days, the permanent pacemaker (PPM) implantation rate was 28.9% (72/249) in all patients and 32.0% (72/225) in pacemaker-naïve patients, mostly (82%) for third-degree atrioventricular block. Among all PPM patients, 28% had right bundle branch block (RBBB) at baseline and 60% had ≥10% LVOT overstretch. Between 30 days and one year, nine additional patients required pacemakers. Mortality at one year was 12.6% (18/143) among patients with no pacemakers compared to 12.3% (10/81, p=0.96) for those with new PPM implantation.
Discussion
The prospective, multicentre REPRISE IIE trial evaluated TAVI outcomes with the LOTUS valve in high-risk surgical patients with severe, symptomatic aortic stenosis. The primary endpoints were met as the observed 30-day mortality rate in the ITT cohort (4.4%, N=250) and mean valve gradient in the main cohort (11.5±5.2 mmHg, N=120) were both below (p<0.001) their respective predefined performance objectives. At one year, there were no cases of ectopic valve deployment, valve embolisation, TAV-in-TAV deployment, valve migration, or repeat procedures for valve-related dysfunction. The eight cases of periprocedural cardiac tamponade probably reflected a learning curve with new operators, given that there were no run-in cases, and the absence of a pre-shaped guidewire – now typically used with LOTUS – in the early trial experience. Improvements in haemodynamics and functional class are similar to outcomes with other transfemoral valves, and were sustained to one year. Post-dilation is typically not recommended with the LOTUS valve and was not performed in this study, though it may be useful if there is significant frame distortion and an elevated gradient. Patients receiving the LOTUS (N=249) had low 30-day and one-year rates of all-cause mortality (4.0%, 11.6%) and disabling stroke rates of 2.8% and 3.6%, respectively. Independent core laboratory adjudication detected very low rates of significant PVL. These REPRISE IIE outcomes support and broaden previously reported results from the main cohort1,2. Notably, these results were obtained with 17/20 centres new to the LOTUS device and all cases were included.
Potential benefits of the LOTUS valve include early valve function to facilitate initial positioning without rapid pacing or haemodynamic instability, full repositionability/retrievability if needed, and minimal PVL. Repositioning was successful in all 85 attempted REPRISE IIE cases. In REPRISE IIE, moderate PVL was negligible (0.6% at 30 days; 0% at one year), with no severe PVL. Mild or greater PVL occurred in only 14.2% of patients at 30 days and 14.7% at one year. This compares favourably with results from other valves, where mild or greater PVL has ranged from 26% to 49% in recent studies of the SAPIEN 3 valve6-8 and from 32% to 50% with the Evolut™ R valve (Medtronic)9-11. Importantly, mild or greater post-procedural PVL has been reported as an independent predictor of mortality12,13.
Although differences in baseline characteristics and variability in endpoint assessment make it challenging to compare results across studies, outcomes in REPRISE IIE were consistent with event rates reported for other contemporary transfemoral TAVI valves in high-risk patients. Per the REPRISE II protocol, neurological physical examinations were conducted by a neurologist or neurology fellow at baseline and prior to discharge. This rigorous neurologic evaluation was expected to result in better stroke detection and may explain differences compared to studies with less rigorous neurologic evaluation6,10. The 30-day PPM rate was 28.9% in all patients and 32.0% in pacemaker-naïve patients with this first-generation LOTUS valve, higher than observed with SAPIEN 36-8 and Evolut R9-11. Mechanical pressure from the valve frame may cause conduction disorders, especially with longer stent frames, self-expanding valves, predilatation or post-dilatation and deeper implant depth14. Higher final LOTUS valve depth may reduce the need for PPM without increasing the risk of PVL, and a greater range of LOTUS valve sizes may reduce overstretch, which was seen in 60% of PPM patients. While undesirable, PPM early after TAVI has generally not been associated with an increase in mortality15-17. Although not statistically powered, one-year mortality rates in REPRISE IIE were similar among patients with or without a pacemaker.
Limitations
The limitations of this study include the absence of a randomised control, which makes it difficult to compare directly the results in this study to results observed with other valves, the moderate sample size, and absence of longer-term follow-up, all of which will be addressed with further follow-up and additional studies. Another limitation is that, although the study was designed to enrol high-risk patients, the mean STS score was 6.5% and some intermediate-risk patients were probably enrolled. This reflects current, “real-world” clinical practice and the challenges in characterising patient risk precisely. Additionally, one-year echo follow-up was only available in 190 of 220 (86%) surviving patients at one year, which is a limitation of the echocardiographic results. Finally, only the 23 mm and 27 mm LOTUS valves were available at the time of the study, which may have affected valve selection and overstretch.
Conclusions
The present study demonstrates the safety and effectiveness of the LOTUS valve in high-risk patients with severe aortic stenosis. The valve could be positioned precisely with minimal PVL and good valve haemodynamics sustained to one year. Significant improvements from baseline in functional status and quality-of-life measures and adverse event rates consistent with those reported for other transcatheter valves were observed. The next-generation LOTUS Edge™ (Boston Scientific) platform with Depth Guard™ technology (Medtronic) is intended to provide improved catheter flexibility, decrease force on the conduction system during delivery, and reduce valve interaction with the LVOT during deployment to facilitate a higher implant. These additional design features are expected to improve deliverability of the valve and reduce the rate of PPM, which will be evaluated in forthcoming trials.
| Impact on daily practice Transcatheter aortic valve implantation is a well-established treatment for severe aortic stenosis in high-risk surgical candidates, and continuous device improvements have sought to widen TAVI application. The REPRISE IIE study demonstrated the safety and effectiveness of the differentiated second-generation fully repositionable LOTUS valve. The LOTUS valve could be positioned precisely with minimal paravalvular leak, and good valve haemodynamics sustained to one year in 250 patients. Significant improvements from baseline in functional status and quality-of-life measures and adverse event rates consistent with those reported for other transcatheter valves were also observed. These results suggest that the differentiated second-generation LOTUS device will be a valuable addition for treatment of severe aortic stenosis. |
Acknowledgements
The authors would like to acknowledge the following investigators and centres that enrolled patients into the study:
Ian T. Meredith, AM, MBBS, PhD (Study Principal Investigator), Monash Medical Centre, Clayton, Victoria, Australia (38); Nicolas Dumonteil, MD, Centre Hospitalier Universitaire Rangueil, Toulouse, France (29); Daniel J. Blackman, MD, Leeds General Infirmary, Leeds, United Kingdom (22); Didier Tchétché, MD, Clinique Pasteur, Toulouse, France (21); David Hildick-Smith, MD, Sussex Cardiac Centre, Brighton and Sussex University Hospitals, Brighton, United Kingdom (19); Ganesh Manoharan, MD, Royal Victoria Hospital, Belfast, United Kingdom (19); Darren Walters, MBBS, The Prince Charles Hospital, Brisbane, Queensland, Australia (19); Jan Harnek, MD, Lund University Hospital, Lund, Sweden (16); Stephen G. Worthley, MD, Royal Adelaide Hospital, Adelaide, Australia (13); Gilles Rioufol, MD, PhD, Hôpital Cardiologique, Hospices Civils de Lyon, Lyon, France (10); Philippe Garot, MD/Thierry Lefèvre, MD, Institut Cardiovasculaire - Paris Sud, Massy, France (9); Arnaud Sudre, MD/Thomas Modine, MD, PhD, CHRU Lille - Hôpital Cardiologique, Lille, France (9); Nicolas Van Mieghem, MD, PhD, Erasmus Medical Center, Rotterdam, the Netherlands (8); Robert J. Whitbourn, MBBS, FRACP, St. Vincent’s Hospital, Melbourne, Victoria, Australia (4); Rüdiger Lange, MD, Deutsches Herzzentrum München, Munich, Germany (4); Simon Redwood, MD, Guy’s and St. Thomas’ NHS Foundation Trust, London, United Kingdom (3); Corrado Tamburino, MD, PhD, Ospedale Ferrarotto, Catania, Italy (3); Ralf Müller, MD, HELIOS Klinikum Siegburg, Siegburg, Germany (2); Eulogio Garcia, MD, Hospital Clinico San Carlos, Madrid, Spain (1); Stephan Windecker, MD, Universitätsspital Bern, Bern, Switzerland (1).
In addition, the authors wish to thank Edmund McMullen, M. Math and Hong Wang, M.Sc., for assistance with statistical analyses, Ruth M. Starzyk, PhD, and Vicki M. Houle, PhD, for assistance with drafting and editing the manuscript, and Blessie Concepcion for critical review of the manuscript (all from Boston Scientific, Marlborough, MA, USA).
Funding
This work was supported by Boston Scientific, Marlborough, MA, USA.
Conflict of interest statement
I. Meredith has received consultant fees and honoraria from Boston Scientific, Elixir, and Medtronic, and proctor fees from Boston Scientific. N. Dumonteil has received consultant fees and honoraria from Biotronik, Boston Scientific, Edwards Lifesciences, and Medtronic, and proctor fees from Boston Scientific, Edwards Lifesciences, and Medtronic. D. Blackman has received consultant fees and honoraria from Boston Scientific and Medtronic, and proctor fees from Boston Scientific. D. Tchétché and J. Harnek have received proctor fees from Boston Scientific. D. Walters has received consultant fees and honoraria from Boston Scientific and Edwards Lifesciences, and grant support from Boston Scientific, Edwards Lifesciences, and St. Jude Medical. D. Hildick-Smith has received proctor fees from Boston Scientific, Edwards Lifesciences, and Medtronic. G. Manoharan has received consultant fees and honoraria from Medtronic and St. Jude Medical, and proctor fees from Boston Scientific. S. Worthley has received consultant fees and honoraria from Medtronic and St. Jude Medical. G. Rioufol has received consultant fees and honoraria from Medtronic and St. Jude Medical. T. Lefèvre has received consultant fees and honoraria from Boston Scientific, Edwards Lifesciences, Sanofi, and Tryton, and grant support from Boston Scientific, Direct Flow, Symetis, and The Medicines Company. T. Modine has received consultant fees from Boston Scientific, Edwards Lifesciences, and Medtronic, and proctor fees from Boston Scientific and Medtronic. N. Van Mieghem has received consultant fees from Boston Scientific and Medtronic, and research grant support from Abbott Vascular, Boston Scientific, Claret Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical. T. Feldman has received consultant fees, honoraria and grant support from Abbott, Boston Scientific, and Edwards Lifesciences. D. Allocco and K. Dawkins are full-time employees and stockholders of Boston Scientific Corporation.

