Since transcatheter aortic valve implantation (TAVI) achieved Conformité Européenne in 2006, both the efficacy and safety of the therapy have improved considerably. Achille’s heels, such as new conduction abnormalities requiring permanent pacemakers, paravalvular leakage (PVL), and vascular complications have consequently become less frequent. Often, these complications are due to the patient phenotype, with aortic root or valvular calcification being a well-recognised risk factor.
In this issue of EuroIntervention, Farhan and colleagues report the outcomes for TAVI in patients with moderate or severe left ventricular outflow tract (LVOT) calcification1. They use data from the SOLVE-TAVI trial2, where patients with severe aortic stenosis were randomised to TAVI with either self-expanding (SE) or balloon-expandable (BE) transcatheter heart valves (THV). Out of 416 eligible patients, about one-third had moderate or severe LVOT calcification. Interestingly, there was no difference in the composite primary endpoint of all-cause mortality, stroke, more than mild PVL, permanent pacemaker implantation, and annulus rupture at 30 days for patients with moderate or severe as compared to patients with no or mild LVOT calcification (Figure 1A). With the exception of new permanent pacemakers, the rates of the individual endpoint components were low. Furthermore, no difference was found for the primary outcomes for SE and BE THV (Figure 1B).
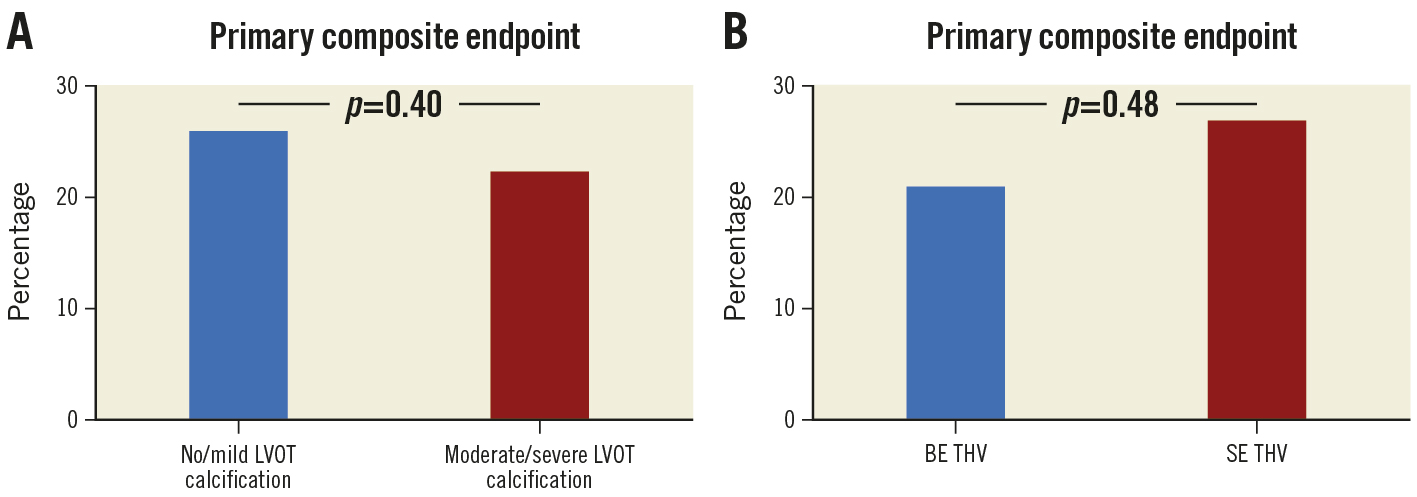
Figure 1. Composite primary endpoint of all-cause mortality, stroke, more than mild paravalvular leakage, permanent pacemaker implantation, and annulus rupture at 30 days for patients with moderate or severe as compared to patients with no or mild left ventricular outflow tract (LVOT) calcification (A), and for self-expanding (SE) and balloon-expandable (BE) transcatheter heart valves (THV) (B).
So, how should we interpretate these new data? TAVI in patients with LVOT calcification has been associated with an increased risk for pacemaker implantation, annulus rupture and PVL. Moreover, annulus rupture is more commonly related to the use of BE THV and aggressive balloon dilatation than with the use of SE THV. Indeed, the introduction of computer tomography (CT) for preprocedural planning has helped to identify patients at risk for procedure-related complications and thereby to tailor the procedure to mitigate the risk of, e.g., annulus rupture.
This raises the question as to whether all patients with severe LVOT calcification undergoing TAVI in the participating institutions were included in the SOLVE-TAVI trial? Since patients had to be deemed suitable for both SE and BE TAVI, is it possible that perhaps some patients were screened out by the enrolling investigators, who felt that the risk of rupture was too high in case of randomisation to BE THV? This may be the case, despite severe LVOT calcification not being an exclusion criterion for study inclusion.
For patients with LVOT calcification who were included in the SOLVE-TAVI trial, the preprocedural CT data would also have led to a more careful strategy for pre- and post-balloon dilatation, and likely the use of smaller balloon diameters, e.g., not exceeding the minor axis of the aortic annulus. Since the burden of PVL after TAVI has decreased with new-generation THV with sealing skirts, there is less need for aggressive balloon pre- or post-dilatation – and, therefore, a lower risk of annulus rupture.
The findings from this study support the impression from daily clinical practice that the complication rates for TAVI have reduced over the years. This is due to a combination of increased operator skills, improved THV designs and delivery systems, and importantly – as mentioned above – comprehensive preprocedural workups including multislice CT. Thus, even patients with excessive calcification of the aortic valve complex, including those with bicuspid aortic valves, can now be treated with TAVI with a low risk if the THV type and size, as well as balloon diameters, are carefully tailored to the individual patient. Clinicians should, however, remain careful when using balloon-expandable valves in heavily calcified LVOT anatomies, since the current study was not a randomised comparison between devices in these patients, but rather a post hoc analysis which should be considered hypothesis-generating. Good clinical judgement remains key to successful TAVI.
Conflict of interest statement
L. Sondergaard has received consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic and SMT. D. Mylotte has received consultant fees and/or institutional research grants from Boston Scientific, Medtronic and MicroPort.
Supplementary data
To read the full content of this article, please download the PDF.

