
Abstract
Aims: The aim of the study was to evaluate the procedural and 30-day results for the repositionable Lotus valve in patients undergoing transfemoral aortic valve implantation in a single-centre experience.
Methods and results: We prospectively enrolled 110 patients with severe symptomatic aortic stenosis (NCT02162069). All procedures were performed without general anaesthesia by the transfemoral approach. Patients were followed for 30 days. Patients received the 23 mm (n=20), 25 mm (n=43) or 27 mm (n=47) Lotus device. Mean oversizing in relation to annulus or left ventricular outflow tract (LVOT) did not differ among groups. There was no residual moderate or severe aortic regurgitation. The rate of mild aortic regurgitation was low at 9.1%. There was no valve embolisation, no need for a second valve and no conversion to surgery. The need for a new pacemaker implantation due to complete (third degree) or type II (Mobitz) second degree atrioventricular block was 24.1%, excluding patients with previously implanted devices. Within 30 days the rates of all-cause mortality and stroke were low.
Conclusions: In patients with severe aortic stenosis, transfemoral TAVI with the repositionable Lotus valve was associated with a high rate of device success, no moderate or severe residual aortic regurgitation, low rates of major vascular complications and mortality within 30 days.
Introduction
Transcatheter aortic valve implantation (TAVI) for treatment of symptomatic patients with severe aortic stenosis was associated with a lower long-term mortality compared with patients undergoing surgical valve replacement1. With first-generation TAVI prostheses, residual paravalvular aortic regurgitation (AR) after TAVI has been identified as a significant independent predictor of acute and long-term mortality2. Furthermore, valve positioning could not be completely controlled, leading in some cases to severe complications such as valve embolisation or the need for a second valve implantation.
The Lotus™ valve (Boston Scientific, Marlborough, MA, USA) incorporates two dedicated design features to obtain optimised post-procedural results. First, the mechanically deployed valve can be repositioned to achieve an optimal valve positioning. Even after final placement in the annulus, the valve can still be completely retrieved, which should eliminate the risk of valve embolisation and the need for implantation of a second valve. Second, an adaptive outer seal minimises residual paravalvular AR (Figure 1). Published data with the Lotus valve do not include the full range of available sizes3-5 and are mainly limited to single case reports6-8.

Figure 1. The Lotus valve. The repositionable, mechanically deployed Lotus valve with an adaptive seal at the outer side of the distal frame to reduce paravalvular aortic regurgitation.
We evaluated periprocedural safety, post-procedural results including residual AR, device success and outcome within 30 days according to the second Valve Academic Research Consortium criteria9 for the repositionable and retrievable Lotus valve in a patient population with symptomatic severe aortic stenosis undergoing transfemoral TAVI.
Methods
We prospectively evaluated the safety and efficacy of the Lotus valve in 110 patients with symptomatic severe aortic stenosis including the 23, 25 and 27 mm valve sizes. Valve implantation was performed in a hybrid catheterisation lab without general anaesthesia by the transfemoral approach as described elsewhere7,8,10. Severe aortic stenosis was documented by echocardiography and cardiac catheterisation with an aortic valve area (AVA) ≤1 cm2 or an indexed AVA ≤0.6 cm2/m2. Patients were at intermediate to high risk for surgical valve replacement based on a Society of Thoracic Surgeons (STS) score for mortality or had relevant comorbidities with contraindications to surgical valve replacement, e.g., porcelain aorta, frailty or history of chest radiation. The Heart Team including cardiologists and heart surgeons made the decision for TAVI. Written informed consent was obtained from each patient. The study was approved by the local ethics committee (ClinicalTrials.gov NCT02162069).
Pre-procedural 256 multislice computed tomography was used for sizing in all patients. Measurements were obtained using dedicated software (3mensio 7.0 software; Pie Medical Imaging, Maastricht, The Netherlands). Aortic cusp calcification was assessed according to Rosenhek11. Eccentricity index was calculated as 1–(minimum diameter of annulus/maximum diameter annulus). Oversizing or undersizing was calculated as % oversizing=(Lotus valve nominal area/annular area by computed tomography–1)*100. Nominal areas for the 23, 25 or 27 mm Lotus valve were 415.5, 490.6 and 572.5 mm2 according to the manufacturer’s instructions for use. The presence of LVOT calcification was defined as moderate or severe as defined by Barbanti et al12.
We studied the full range of available Lotus sizes including 23, 25 and 27 mm devices which allowed us to address the outcome of the Lotus valve in a general TAVI population. The 23 mm (25 mm; 27 mm) Lotus valve size was implanted in patients with a diameter of the annulus between 20 and 23 mm (23-25 mm; 25-27 mm). In addition, the distance between annulus and coronary ostia had to be >10 mm and the femoral and iliac arteries had to accommodate the 18 or 20 Fr delivery sheath. Puncture of the femoral artery was performed under fluoroscopic guidance during antegrade angiography. The femoral access was pre-closed with two ProGlide devices (Abbott Vascular, Santa Clara, CA, USA). The Lotus valve was implanted under fluoroscopic guidance without rapid pacing after predilation as described elsewhere8,10. Appropriate valve positioning was achieved by positioning the posts (distal part of the locking mechanism) over a predetermined landing plane.
AR after TAVI was analysed by standardised aortography10,13. Aortography for AR assessment was performed after complete release of the Lotus valve and after withdrawal of the Safari™ wire (Boston Scientific) by a pigtail catheter from the left ventricle. In all cases, the pigtail catheter for aortography was placed directly on top of the Lotus valve. AR and measurement of pressure gradients by transthoracic echocardiography (TTE) was carried out one to three days after TAVI. AR was graded none, trace, mild, moderate or severe as described elsewhere10,14,15 and differentiated into valvular and paravalvular. Post-procedural TTE was performed by cardiologists specialised in non-invasive imaging. Operators implanting the Lotus valves did not perform post-procedural TTE.
Post-procedural outcome was analysed according to the VARC-2 criteria9. Patients were followed for 30 days.
Statistical analysis
For statistical analyses, the Statistica software, version 10 (StatSoft, Inc., Tulsa, OK, USA) was used. Pre-procedural data and post-procedural results were compared among the three different valve sizes. The primary outcome measure was device success according to VARC-2 criteria, defined as the absence of procedural mortality and correct positioning of a single prosthetic heart valve into the proper anatomical position and intended performance of the prosthetic heart valve. Secondary endpoints were all-cause mortality at 30 days, disabling and non-disabling stroke, life-threatening bleeding, major vascular injury and new pacemaker insertion due to second degree (type II) or third degree atrioventricular block. All endpoints were defined according to VARC-2 criteria. Continuous variables are expressed as mean±one standard deviation and were compared with ANOVA testing. Categorical variables are presented as counts and percentages and differences between proportions were calculated by using the chi-squared test. A value of p<0.05 was considered statistically significant.
Results
Between January 2014 and July 2015 the Lotus valve was successfully implanted in all 110 patients. Baseline, procedural and follow-up data at 30 days were available for all patients. Patients had multiple comorbidities including porcelain aorta, pulmonary disease or reduced left ventricular function, as displayed in Table 1. The American Society of Anaesthesiologists score for perioperative risk was 3.6±0.6; the STS predicted risk of operative mortality was 6.5±4.6 and 15.5±14.1 by logistic EuroSCORE estimates. Mean EuroSCORE II was 7.0±7.4% with 82 (74.5%) patients being at intermediate risk. There was no difference among groups regarding the number of patients who were at high or intermediate risk based on EuroSCORE II (23 mm Lotus valve 75.0%; 25 mm valve 67.4%, 27 mm valve 80.9%; p=0.34). A history of cardiac surgery was present in 10% and atrial fibrillation in 38% of patients. The majority of patients (82%) were severely symptomatic with NYHA Class III or IV. There was no difference regarding baseline clinical data or baseline aortic valve parameters among the 23, 25 or 27 mm population (Table 1, Table 2).
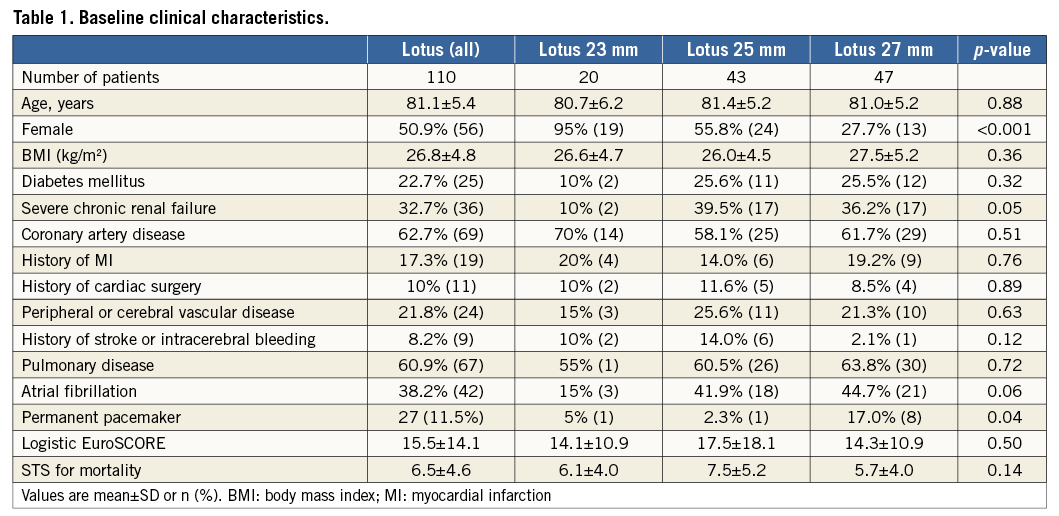
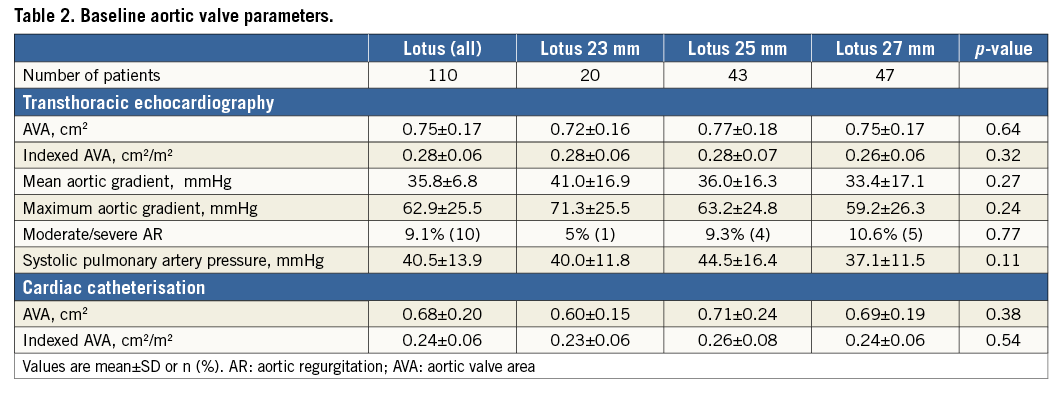
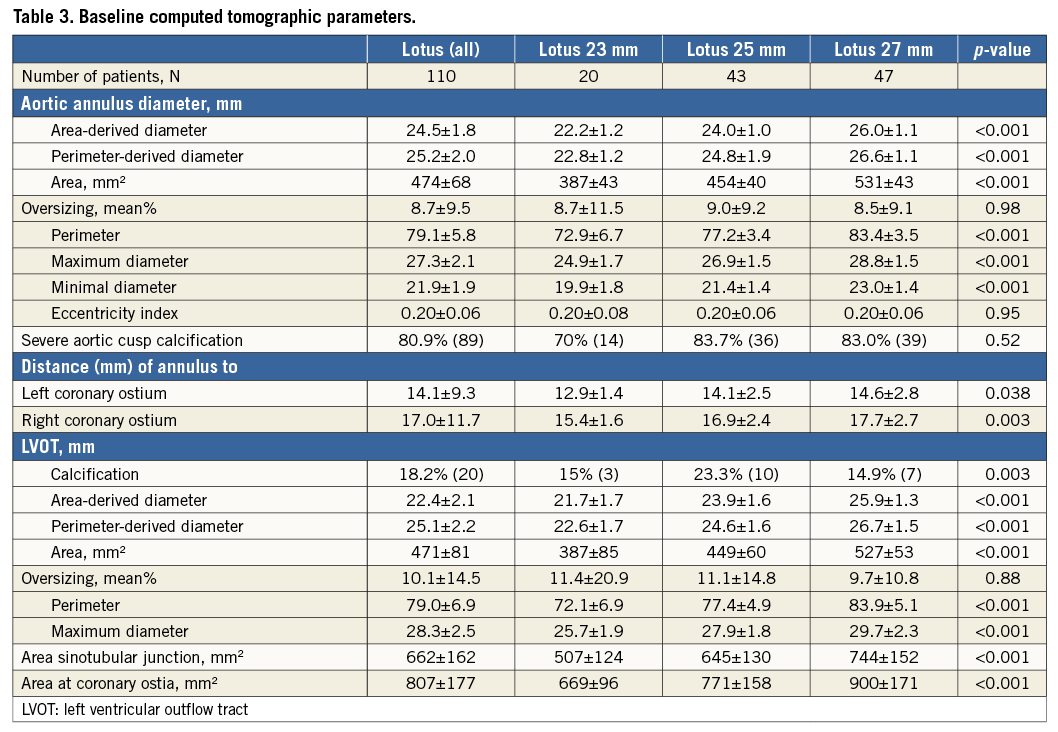
Measurements of the annulus and LVOT parameters were significantly different for the three valve sizes with a perimeter-derived diameter of the aortic annulus of a mean of 22.8 mm for the 23 mm group (n=20), 24.8 mm for the 25 mm group (n=43) and 26.6 mm for the 27 mm group (n=47) (Table 3). The distance from the annulus to the left or right coronary ostium was shortest in the 23 mm population and longest in the 27 mm group. There was no difference in the rate of oversizing in relation to the annulus among the three valve sizes, ranging from 8.5% to 9.0%. In addition, there was no difference in the rate of oversizing in relation to the LVOT, ranging from 9.7% to 11.4% (Table 3). Calcifications of aortic cusps and LVOT calcifications were similar among the three groups.
PROCEDURAL RESULTS AND FOLLOW-UP
The Lotus valve was successfully implanted in all 110 patients by transfemoral access without general anaesthesia. Due to the option to reposition the Lotus valve, there was no need for a second valve and no valve embolisation. Partial resheathing was used in 21.8% and complete resheathing in 11.8% of patients. There was no procedural death, no coronary obstruction, annular rupture or need for conversion to surgery. Moderate or severe AR after valve release in the final position was completely absent in all three groups assessed by aortography and echocardiography (Table 4). On TTE after TAVI, the rate of mild AR was 9.1% (one valvular, nine paravalvular). There was a significantly higher rate of mild AR in patients with LVOT calcification compared to patients without calcification of the LVOT (25% versus 5.6%, p<0.01), as detailed in Figure 2. The residual mean aortic gradient by echocardiography did not differ among groups and was a mean of 12.1±4.6 mmHg. The rate of device success was high at 95.5%, with no difference among groups (Table 4). All patients without device success had slightly elevated mean aortic gradients >20 mmHg as assessed by echocardiography (22 mmHg [n=2], 23 mmHg [n=2] or 32 mmHg [n=1]). In patients with device failure, there was no difference with respect to oversizing in relation to annulus or LVOT, LVEF, eccentricity index or presence of LVOT calcification compared with patients with device success.
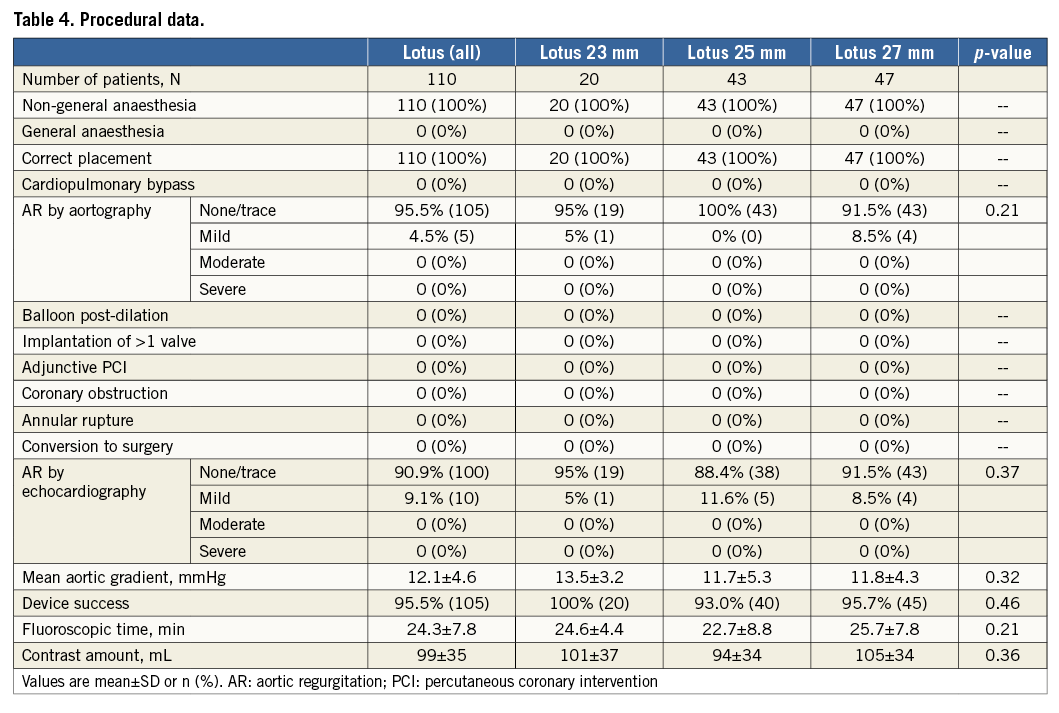
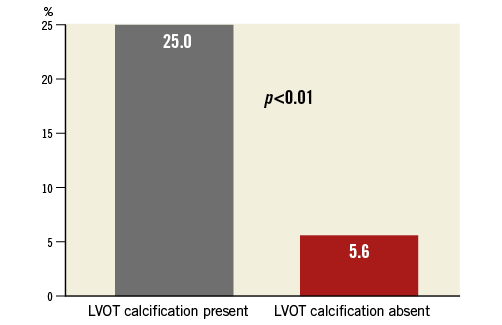
Figure 2. Post-procedural mild aortic regurgitation. There was a significantly higher rate of post-procedural mild aortic regurgitation in relation to calcification of the left ventricular outflow tract.
The rate of major vascular complications was 4.5% with no need for surgical repair. All five cases with major vascular complications were related to closure device failure in combination with a drop in haemoglobin of more than 3 g/dl. Although there was no statistical difference regarding the occurrence of major vascular complications among groups, all five patients with major vascular complications were treated with the 25 or 27 mm Lotus valve requiring the larger sheath.
The need for new pacemaker insertion due to type II (Mobitz) second degree atrioventricular block or complete (third degree) atrioventricular block was 18.1% and did not differ among groups. Implantation depth did not differ between patients with and without the need for permanent pacemaker implantation. Median oversizing in relation to the annulus was 7.6% and median oversizing in relation to LVOT 10.8%. For both calculations of oversizing, the need for pacemaker implantation was 21.8% (n=12/55) for patients below the median versus 14.6% (n=8/55) for patients above the median (p=0.32). There was a significantly higher need for pacemaker implantation in patients with calcification in the LVOT compared to patients without LVOT calcification (35.0% versus 14.4%, p=0.03) (Figure 3). The need for permanent pacemaker implantation in patients without a permanent pacemaker before TAVI was 24.1% (n=20/83).
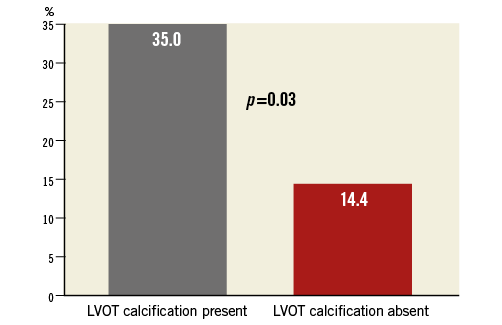
Figure 3. Need for permanent pacemaker implantation. The need for permanent pacemaker implantation was significantly higher in patients with calcification of the left ventricular outflow tract compared with patients without calcification of the left ventricular outflow tract.
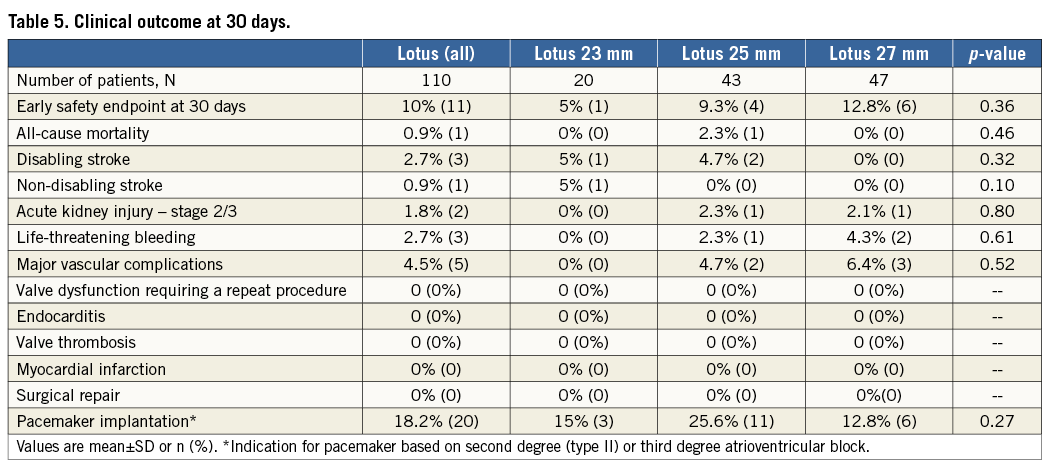
The early safety endpoint at 30 days was met in 10% and was similar among groups (Table 5), with no difference in the rates of all-cause mortality (0.9% for total population) or disabling (2.7% for total population) and non-disabling stroke (0.9% for total population). Three out of four strokes occurred within 24 hours after valve implantation; the fourth stroke occurred three days after the procedure.
Discussion
We were able to demonstrate a high rate of device success and low rates of mortality and stroke within 30 days according to VARC-2 criteria for the Lotus valve in symptomatic patients undergoing transfemoral TAVI. With all valve sizes, there was no residual moderate or severe paravalvular AR, with a similar rate of oversizing in relation to the annulus and LVOT. Based on the option to reposition the Lotus valve, there was no valve embolisation and no need for a second valve.
Residual AR after TAVI is a significant independent predictor of long-term mortality2,16. The risk of moderate or severe AR with balloon-expandable TAVI prostheses ranged between 5 and 10%15-17, and was up to 18% for self-expanding TAVI prostheses even after post-dilation15. In early experiences with the Lotus valve, moderate AR was reported in up to 4.0%3-5,10. The Lotus valve has been designed to eliminate post-procedural AR by an adaptive seal and by the unique delivery technique, which makes it possible to reposition and to completely retrieve the valve18. We report a single-centre experience including the full available range of valve sizes (23, 25 and 27 mm), demonstrating the elimination of moderate or severe residual AR for this first-generation repositionable TAVI device. In addition, there was no need for post-dilation. In the very first Lotus experience using the 23 mm device, the rate of moderate or severe AR in 11 patients was 0% and the rate of mild AR 18.2%3. In the REPRISE II CE mark multicentre study including 120 patients treated with the 23 and 27 mm device, the rate of paravalvular moderate or severe AR at discharge was 2.0% and the rate of mild AR 13.1%4. Numbers were 2% and 6% in a single-centre experience including 50 Lotus cases with 23 and 27 mm devices, respectively5. We observed mild AR in 9.1% with no difference among the three valve sizes, with a similar rate of oversizing in relation to the annulus and LVOT. There was a significantly higher rate of mild AR in patients with LVOT calcification. The presence of LVOT calcification has been shown to be an independent predictor of moderate AR in patients undergoing TAVI with other valve types12,19.
Device success with the Lotus valve was high at 95.5%. All patients without device success showed an elevated mean aortic pressure gradient on echocardiography. All valves were correctly positioned and there was no procedural mortality or cardiac perforation. There was no conversion to surgery and no need for a second valve. In other Lotus valve series, device success was similar at 95.3%4 and 96%10, except for a single-centre experience reporting 84% in 50 patients5.
The need for pacemaker implantation with the Lotus valve was 36% in REPRISE I (23 mm devices) and 29% in REPRISE II (23 and 27 mm devices). The indication for pacemaker implantation was third degree atrioventricular block in 88% of patients in REPRISE II, resulting in a need for pacemaker implantation due to total atrioventricular block of 25.2% for the total population. With three Lotus valve sizes available, the need for pacemaker implantation due to second (type II) or third degree atrioventricular block was 24.1% in our population, excluding patients with previously implanted devices. The presence of LVOT calcification significantly correlated to the need for pacemaker implantation. The need for pacemaker implantation with the Lotus valve was higher compared with balloon-expandable valves10,17,20,21 but similar to the rates reported with self-expanding valves15,22. The need for pacemaker implantation was not associated with long-term sequelae22,23. However, a higher need for permanent pacemaker implantation with the repositionable Lotus valve in contrast to balloon-expandable aortic valves could have a negative impact in younger patients or in patients with reduced left ventricular function.
The low mortality rate of 4.2% within 30 days observed in REPRISE II4 was confirmed in our population. The rate of disabling and non-disabling stroke was 3.6% in 110 patients and lower compared to the rate of 5.9% in REPRISE II and of 4.9% in the CoreValve High Risk Study1. Three out of four strokes occurred within 24 hours after valve implantation. Whether the overall stroke rate can be reduced by the use of protection devices has to be studied further.
Patients treated with the 23 mm Lotus valve were predominantly female with a low rate of diabetes mellitus. The presence of diabetes mellitus in female patients may limit the use of the 23 mm Lotus valve due to the need for an 18 Fr delivery sheath. In REPRISE II, the rate of major vascular complications was lower at 2.5% in 120 patients4 in contrast to 4.5% in our population of 110 patients. The use of the 25 and 27 mm Lotus valve was more frequent in our population at 82% compared with about 50% in REPRISE II. For implantation of the 25 and 27 mm Lotus valve, the diameter of the sheath is larger compared to the size for implantation of the 23 mm Lotus valve. The higher usage of the larger sheath in our population in contrast to REPRISE II could have had an impact on the occurrence of major vascular complications due to a higher risk for closure device failure.
Limitations
There was no use of embolic protection to reduce the risk of periprocedural cerebral embolic events. There was no external echo core lab, although cardiologists specialised in non-invasive imaging not performing TAVI procedures performed TTE.
Conclusions
In patients with severe aortic stenosis, transfemoral TAVI with the repositionable Lotus valve was associated with a high rate of device success, no moderate or severe residual aortic regurgitation, low rates of major vascular complications, mortality and stroke within 30 days.
| Impact on daily practice Transfemoral aortic valve implantation with the repositionable Lotus valve is associated with a high rate of device success. With the use of the Lotus valve there is a low risk of moderate or severe residual aortic regurgitation due to the option to reposition and even retrieve the valve. In addition, there was no need for a second valve and no conversion to surgery. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

