
Abstract
Aims: To demonstrate the feasibility of implanting the Lotus second-generation transcatheter heart valve (THV) (designed for a transfemoral procedure) via a transaortic approach.
Methods and results: We describe a case with severe aortic stenosis in the presence of small calibre and calcified femoral access and severe chronic obstructive pulmonary disease. The transaortic approach was the ideal approach for this patient and we successfully implanted a 25 mm Lotus valve without any complication.
Conclusions: The transaortic access is a feasible and safe alternative in patients who have suboptimal iliofemoral conduits and who will benefit from the unique features of the Lotus THV.
Introduction
Transcatheter aortic valve implantation (TAVI) has now become an established treatment in patients with calcified aortic stenosis who are inoperable or high risk for conventional aortic valve replacement. Multiple transcatheter heart valve (THV) types are now available for implantation. The transfemoral (TF) route is the dominant access route with the transapical (TA) the most common alternative. Subsequently, with the focus on minimising patient morbidity and mortality, alternative access routes, namely the transaortic (TAo) and subclavian (TS), have been developed. The Lotus™ valve (Boston Scientific, Marlborough, MA, USA) is a second-generation THV device with two unique features: it is repositionable and retrievable, and has an adaptive seal to reduce paravalvular leak. It is implanted using a dedicated TF delivery system, which has a “precurve” to accommodate the curve of the aortic arch. Early clinical studies have shown good short-term results1. As the TF approach is not suitable in all patients, our aim was to explore the feasibility of the TAo approach for Lotus THV implantation using the existing TF delivery system. Here we report our first-in-man experience in TAo implantation of the Lotus valve with emphasis on case planning, lab set-up and procedural tips.
Case report
An 82-year-old lady was suffering with increasing shortness of breath (NYHA functional Class III), exertional pre-syncope and chest pain (CCS Class I). Clinical and echocardiographic assessment confirmed severe aortic stenosis with a valve area of 0.5 cm2, and preserved left ventricular systolic function with an estimated ejection fraction of 65%. Other history included a cerebrovascular accident with some residual left-sided weakness, paroxysmal atrial fibrillation, and chronic obstructive pulmonary disease with a forced expiratory volume in one second (FEV1) of 0.8 L (0.64%). Further workup included a carotid duplex scan, invasive coronary angiography, contrast-enhanced CT of the aorta and peripheral vasculature. There were no flow-limiting coronary lesions but the iliofemoral vessels were small, tortuous, and with extensive calcification.
The patient was assessed by two cardiac surgeons and judged to be at prohibitively high risk for surgical aortic valve replacement (SAVR). She was therefore discussed by the TAVI Heart Team and deemed suitable for TAo TAVI, to be performed as a first-in-man using the Lotus valve.
Lotus valve
The Lotus THV is a second-generation self-expandable valve available in 23 mm, 25 mm and 27 mm diameter sizes. It consists of three bovine pericardial leaflets attached to a braided nitinol frame with a radiopaque marker used for accurate placement. The valve is designed to expand radially as the valve shortens during deployment. Rapid pacing is not required during the implantation.
Delivery system
The valve is delivered using a delivery system which is introduced through a dedicated sheath (20 or 22 Fr in outer diameter). As the precurve in the delivery system, which was designed to facilitate the TF approach, cannot be overcome with any available sheath, it is important to choose an ideal site for the aortic puncture so as to achieve a coaxial placement of the Lotus valve (Figure 1A). The site chosen in this case was at least 6 cm from the aortic annulus and was on the anterior wall of the ascending aorta (Figure 1B, Figure 1C).
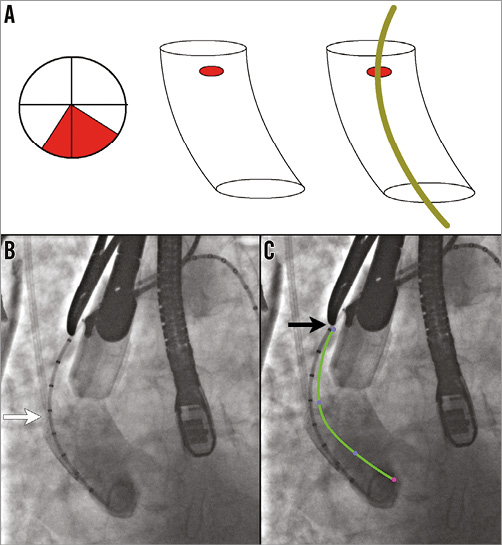
Figure 1. Site of aortic access and purse-string suture position. A) The TAo zone or the ideal site for purse-string sutures is in the anterior wall of the ascending aorta to accommodate the precurve in the sheath. B) Aortogram identifying the valve deployment view as well as confirming the distance between the aortic annulus and site of purse-string sutures (at least 6 cm). White arrow points to calibrated pigtail and black arrow (in panel C) points to the metal marker at the proposed site of purse-string sutures. C) Superimposition of the precurve of the delivery system.
Procedural steps
Exposure was obtained via a mini-sternotomy through a 2nd right intercostal space (Figure 2A). After dissecting the mediastinal fat, the pericardium was opened and the ascending aorta identified. A spot free of calcification on either the anterior or left side of the ascending aortic wall was identified and two purse-string sutures were placed to accommodate the sheath (Figure 2B). A pigtail catheter (INFINITI®; Cordis Corp., Fremont, CA, USA) was placed through the femoral artery and an aortogram performed to identify a deployment view and to confirm the distance between the aortic annulus and the site of the purse-string sutures (Figure 1B, Figure 1C). This has been described previously2. Access to the aorta was obtained by a needle puncture, which was later replaced with a 6 Fr sheath. A multipurpose catheter and straight 0.014” wire were used to cross the aortic valve, and a Safari™ pre-shaped TAVI guidewire (Boston Scientific) with a short loop exchanged into the ventricle. The 6 Fr sheath was then replaced with the sheath from the Ascendra delivery system (Edwards Lifesciences, Irvine, CA, USA), which was found to be the most suitable for this procedure. Care was taken to place the sheath 2-3 cm inside the aorta. A balloon aortic valvuloplasty (BAV) was performed with a 20 mm balloon (VACS II balloon; OSYPKA AG, Rheinfelden, Germany). The delivery system was then inserted in such a way that the precurve of the delivery system aligned exactly with the curve of the aorta (Figure 3). A 25 mm valve was then deployed without rapid pacing. During deployment, the sheath was managed carefully so as to avoid either pop-out or deep placement, which would have prevented complete unsheathing of the device (Figure 4, Moving image 1). A final check for coronary obstruction and PV leak was performed with TOE and aortography. The delivery system and sheath were then removed and the purse-string sutures closed. After haemostasis, a pericardial drain was placed and the chest closed with four sternal wires. The patient was extubated on the table after the procedure.
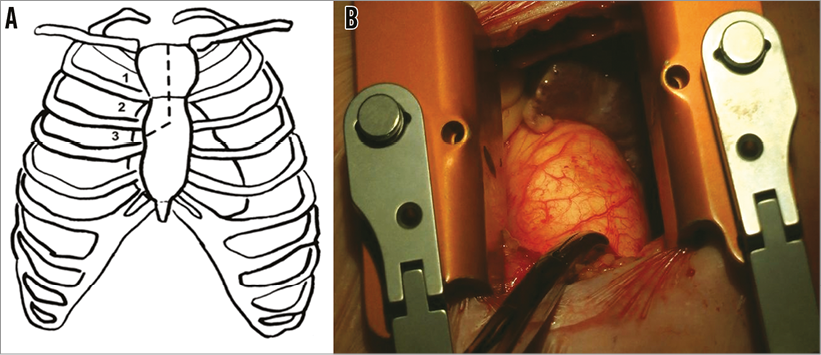
Figure 2. Sternotomy site and operative view. A) Exposure was obtained via a mini-sternotomy through a 2nd or 3rd right intercostal space. The dotted line in the left-hand panel shows the access schematic. B) Operative exposure with metal marker at the site of purse-string sutures.
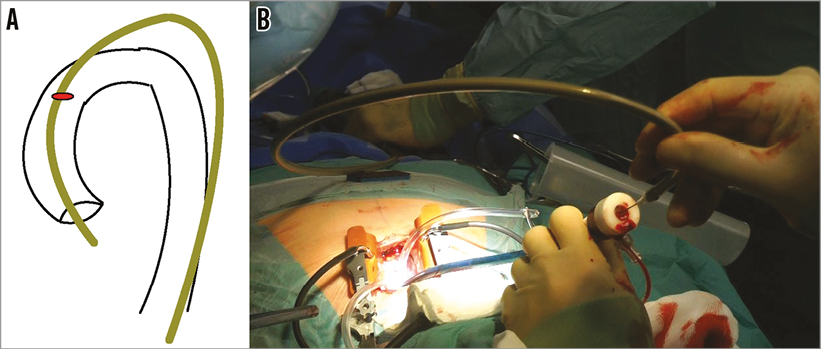
Figure 3. Alignment of delivery system and aortic curvatures. Due to the delivery system precurve, which cannot be straightened, the valve is best deployed by simulating the transfemoral route but outside the body. A) Schematic diagram of the lie of the delivery system. B) Operative picture demonstrating the set-up and lie of the sheath and delivery system.
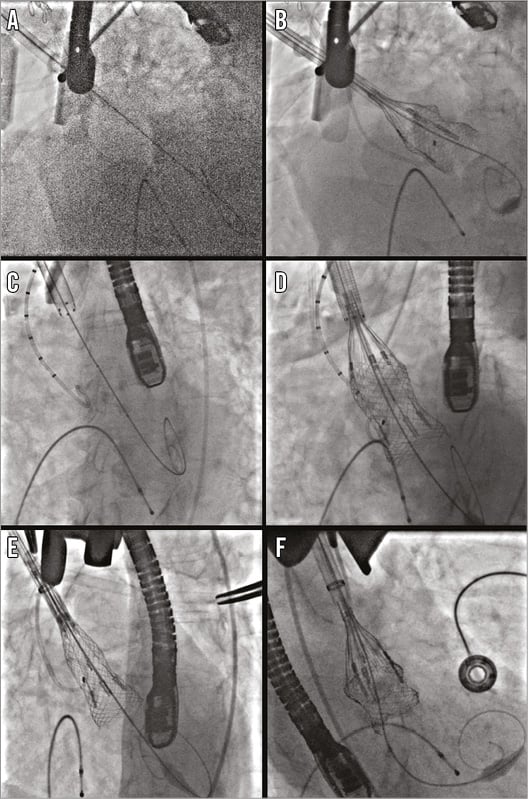
Figure 4. Sheaths, positioning and valve deployment. Various sheaths have been trialled for TAo Lotus valve deployment. A) & B) The Lotus Introducer Set has no tip or external marker, and is hydrophilic. C) & D) The Edwards Ascendra+ sheath has a marker at the tip, and the external marker is non-hydrophilic. E) & F) The GORE DrySeal sheath has a marker at the tip but no external marker and is hydrophilic.
Successful implantation was achieved, as defined by VARC-2 criteria. Procedure time was 94 mins, with fluoroscopy time of 16.5 mins. The total contrast volume used was 100 ml. There were no significant procedural complications and the patient was successfully transferred to the intensive care unit. Sinus rhythm with left bundle branch block, which was new, was the post-procedure rhythm.
Post-procedure outcome
The postoperative course was unremarkable. There were no higher degrees of atrioventricular block throughout the in-patient stay and therefore a permanent pacemaker was not required. Renal function remained normal. The patient was discharged home on day nine post procedure. A pre-discharge echocardiogram demonstrated a calculated valve area of 1.4 cm2, with normal left ventricular function. At 30-day follow-up the patient was NYHA Class I and CCS 0, with no change in valvular haemodynamics.
Discussion
This case represents the first-in-man experience of TAo implantation of the Lotus THV using the first-generation delivery system.
The TAo approach is now a well-established approach for TAVI and, theoretically, using the approaches described here, any TAVI device which can be implanted through a TF approach can be used through a TAo approach. The feasibility of the TAo approach will be dictated by the type and design of the device and the configuration of its delivery system. Most delivery systems which have been used for the TAo approach are either purpose-made, e.g., Ascendra+ and Certitude (Edwards Lifesciences), or flexible, e.g., CoreValve® (Medtronic, Minneapolis, MN, USA) delivery system. It is also important to determine the distance of the aortic purse-string sutures from the aortic annulus so as to allow delivery of the device. For example, 6-7 cm is needed for the SAPIEN valve (Edwards Lifesciences) and CoreValve depending on the size of the device. The Lotus TF delivery system has a nose cone but is unique as it has a rigid precurve. The Lotus valve is delivered through a sheath and, when crimped, is a tubular rigid structure at the distal end of the delivery system. As the valve is deployed, it will reduce in height (Figure 4). We carried out bench testing to determine the minimum distance required, found to be 5 cm. In addition, we had to add 1-2 cm for the sheath tip to be placed within the aorta, hence a minimum distance of 6-7 cm was thought to be mandatory. The site of the purse-string sutures was also different from that for the SAPIEN and CoreValve. For the SAPIEN and the CoreValve, the preferred site of the purse-string sutures would be a lateral portion of the aorta to get a coaxial alignment of the device to the annulus. However, due to the precurve, for the Lotus the purse-string sutures were placed anterior or slightly to the left of the ascending aorta. After insertion of the sheath and the delivery system, the precurve was aligned externally in such a way that the curve matched the curve of the ascending aorta to get a coaxial alignment of the device. In bench testing, we tried three different sheaths, i.e., Lotus introducer sheath (Boston Scientific), Ascendra+ (Edwards Lifesciences), and GORE® DrySeal (W.L. Gore & Associates, Inc., Flagstaff, AZ, USA). None of the sheaths was able to straighten the precurve. The Ascendra sheath was deemed best as it has internal (tip) and external distance markers and it is not hydrophilic (Figure 4). A fluoroscopic marker at the tip of the delivery system is important as it allows the operator to visualise how far the tip is in the aorta and what its relationship is with the delivery system so as to allow complete expansion of the device and avoid pop-out. This was found particularly important for Lotus deployment as the sheath tends to move inwards in the first half of the deployment and outwards during the latter.
The design of a specific TAo device for the Lotus should refine the procedure even more. However, until these devices are brought to the market, the use of the existing TF delivery kit, using the techniques described in this report, allows the Lotus second-generation THV to be a viable treatment option for a large patient population not having suitable femoral anatomy.
Conclusion
The TAo approach, using the Lotus system designed for TF approach, is feasible and safe. The TAo approach mimics the TF approach but allows better fine control over device deployment.
| Impact on daily practice The transaortic approach for delivery of the Lotus THV is safe and feasible. Patients who would benefit from the unique features of the Lotus THV and who have small iliofemoral vasculature could benefit from this treatment modality. |
Conflict of interest statement
V. Bapat is a consultant for Edwards Lifesciences, Medtronic, and St. Jude Medical. S. Redwood and J. Hancock are consultants for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Important steps during deployment of the Lotus valve through the TAo access.

