
Abstract
Percutaneous placement of transcatheter heart valves for treatment of degenerated surgical valves in the aortic and mitral position is an emerging therapy for selected high-risk patients. Here we describe in detail the first case in the literature of a patient (female, 72 years old, log EuroSCORE 22.9%) with a degenerated biological mitral prosthesis which was successfully treated by transapical implantation of a Lotus valve. The case described demonstrates the very controlled feasibility of valve-in-valve treatment for a degenerated mitral bioprosthesis with a mechanically expanding Lotus valve.
Introduction
Following the successful introduction of transcatheter aortic valve implantation (TAVI), the valve-in-valve (ViV) concept, as a treatment for a failing biological aortic as well as mitral prosthesis, is quickly emerging. So far, only balloon-expandable valves have been used for treatment of degenerated surgical mitral valves and surgical annuloplasty rings1-4. Thus, we were intrigued to use the controlled expandable/retrievable Lotus™ valve (Boston Scientific, Marlborough, MA, USA) for the first time for transapical treatment of a degenerated Hancock II® prosthesis (Medtronic, Minneapolis, MN, USA) in the mitral position.
First-in-man case report
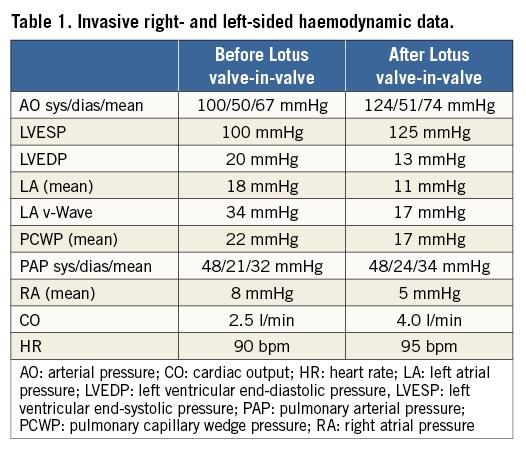
A 72-year-old female patient was referred to our hospital for decompensated heart failure. She had a history of mitral valvular endocarditis treated by mitral valve replacement (Hancock II, 27 mm; Medtronic) in May 2004. In recent months she experienced progressive deterioration of her functional capacity with repeat in-hospital admissions for acute heart failure. On admission she had severe dyspnoea (New York Heart Association [NYHA] functional Class III-IV). There was no history of fever or other signs of recurrent infective endocarditis. Transthoracic (TTE) and transoeosophageal (TEE) echocardiography revealed severe mitral regurgitation (Figure 1A) with a mean transvalvular gradient of 10 mmHg (peak gradient 24 mmHg) and an enlarged left atrium with almost preserved systolic left ventricular function. Measurement of the inner diameter of the prosthetic valve revealed 23.5 mm by TEE and 24.5 mm by multislice computed tomography (Figure 1B). Significant coronary artery disease was ruled out by diagnostic coronary angiography, and dye injection in the left ventricle confirmed severe mitral regurgitation (Figure 2, Moving image 1). The logistic EuroSCORE I was calculated to be 22.9% (STS score risk of mortality: 5.1%). In addition, she had a past medical history of breast and colorectal carcinoma with no signs of recurrent cancer, a primary biliary cirrhosis and severe pulmonary hypertension (sPAP: 65 mmHg). Taken together, the patient was considered at prohibitive risk for repeat open heart surgery by mutual consensus of the local interdisciplinary Heart Team. After informed consent was obtained, the procedure was performed under general anaesthesia. Transapical access was obtained in a minimally invasive, non-rib-spreading fashion via 4 cm skin incision and left anterolateral mini-thoracotomy through the 6th intercostal space. In addition, a Swan-Ganz catheter was introduced in the pulmonary artery. The bioprosthesis was crossed and a Safari™ 260 cm wire (Boston Scientific) was introduced into the left atrium. The 20 Fr sheath was inserted across the mitral bioprosthesis and a 25 mm Lotus valve5 was positioned across the degenerated bioprosthesis without the need for rapid ventricular pacing or preceding balloon valvuloplasty (Figure 3A-Figure 3C, Moving image 2-Moving image 6). The tantalum marker of the Lotus valve was kept constantly 3-4 mm ventricular from the stented ring of the Hancock II prosthesis (Figure 3); no recapture was necessary with this approach. Invasive online monitoring of the haemodynamics revealed stable blood pressure without any sign of haemodynamic compromise (Figure 4). After ruling out any LVOT obstruction, the new bioprosthetic valve was deployed and echocardiography (Figure 5, Moving image 7) as well as ventriculography (Figure 6, Moving image 8) showed complete resolution of mitral regurgitation. Furthermore, fluoroscopy revealed optimal device placement with anatomically correct orientation inside the Hancock II prosthesis (Moving image 5). Invasive right and left-sided haemodynamic measurements showed immediate improvement after transcatheter mitral valve-in-valve implantation (TMViVI) (Table 1). The apical and femoral artery access sites were closed with standard techniques. The procedural time and fluoroscopy time were 78 minutes and 32.1 minutes, respectively. The patient was discharged on postoperative day 13 in NYHA functional Class II on aspirin 100 mg, clopidogrel 75 mg and warfarin for four weeks, followed by oral anticoagulation alone. At 30 days, the patient was largely asymptomatic (NYHA Class II) and echocardiography showed persistent abolishment of any leak with a mean transvalvular gradient of 6-7 mmHg, and preserved left ventricular function (LVEF 55%).
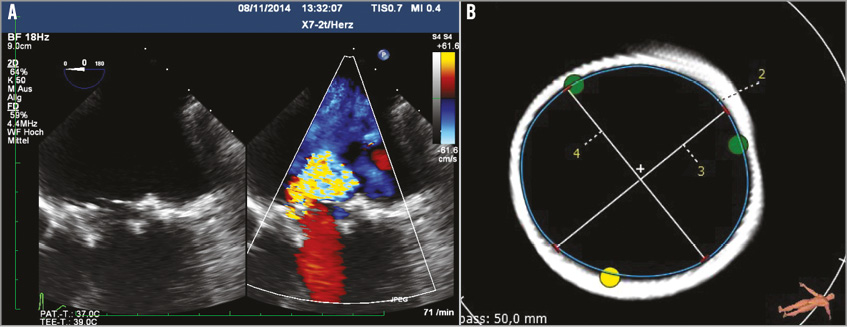
Figure 1. Dysfunctional Hancock II valve. A) Transoeosophageal echocardiography (TEE) revealed severe mitral regurgitation. B) Measurement of the inner diameter of the prosthetic valve revealed 23.5 mm by TEE and 24.5 mm by MSCT.
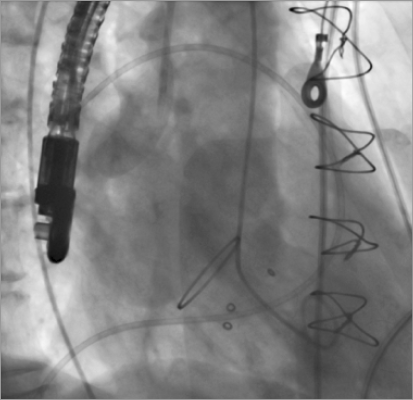
Figure 2. Left ventricular angiography confirmed severe mitral regurgitation.
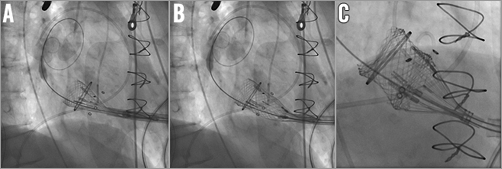
Figure 3. A 25 mm Lotus THV is positioned across the degenerated bioprosthesis without the need for rapid ventricular pacing or preceding balloon valvuloplasty. A)-C) Implantation steps.
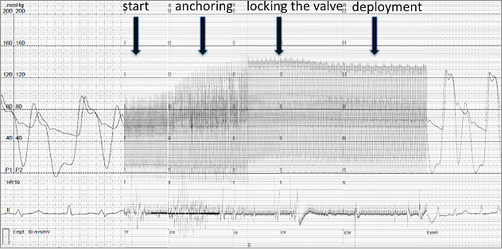
Figure 4. Invasive online recording of the haemodynamics (simultaneous monitoring of left ventricular pressure and aortic pressure, bigeminus), without any sign of haemodynamic compromise. Note: there is an immediate increase in blood pressure once the valve is anchored and there is no left ventricular outflow tract obstruction.
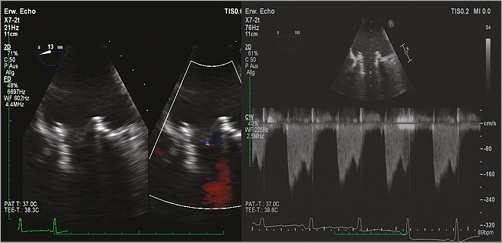
Figure 5. Transoeosophageal echocardiography (TEE) demonstrated complete resolution of mitral regurgitation after implantation of the Lotus THV inside the Hancock II prosthesis.
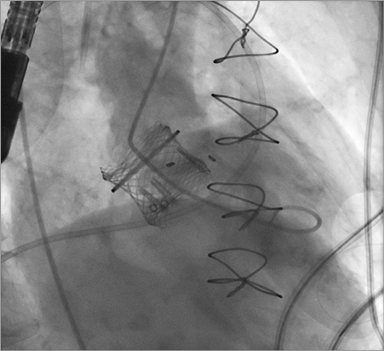
Figure 6. Ventriculography showed a complete resolution of mitral regurgitation with an anatomically correct orientation inside the Hancock II prosthesis.
Discussion
The feasibility of transcatheter mitral valve-in-valve implantation (TMViVI), although considered as off-label use, has been repeatedly demonstrated in the past, providing significant clinical improvement1-3.
Despite the suboptimal initial results with TMViVI (in-hospital mortality 28.6%4 and 33.3%1), transapical TMViVI has been repeatedly proposed as offering an alternative and safe approach for high-risk redo surgical patients. Indeed, within the two largest published series, all patients had been successfully treated with no 30-day mortality2,3. Most of the reported haemodynamic characteristics (obtained almost exclusively with Edwards SAPIEN THV) were acceptable with resultant mean gradients ranging from 5.0 to 8.0 mmHg and mitral surface areas between 1.7 and 2.2 cm22,3. A major uncontrollable disadvantage with the use of balloon-expandable valves (“single shot device”) is the risk of device embolisation or left ventricular outflow tract obstruction. Indeed, Cerillo and colleagues reported a series of three patients with failing mitral bioprostheses (all CE PERIMOUNT 25 mm [Edwards Lifesciences, Irvine, CA, USA], treated by transapical ViV implantation with Edwards SAPIEN THV 26 mm) in whom conversion to open heart surgery became necessary due to migration of the SAPIEN THV into the left ventricular outflow tract (LVOT) with subsequent severe subaortic obstruction1.
In contrast to balloon-expandable THV, the very controlled implantation with a Lotus valve5 can prevent a suboptimal implantation, since the device can be re-sheathed and repositioned in case of malpositioning. A valuable advantage of the Lotus valve is the fact that LVOT obstruction can be ruled out at any time of the procedure before final deployment is performed. In addition, due to the lower stent height of the Lotus valve (all sizes: 19 mm) compared to SAPIEN 3 valves, interference with the LVOT might be less likely to occur. In this regard, the lower stent height of the SAPIEN XT is advantageous (23 mm valve: 18 mm; 26 mm valve: 20 mm; 29 mm valve: 22.5 mm). Finally, due to the adaptive seal of the Lotus valve, complete abolishment of mitral regurgitation with acceptable transvalvular gradients can be achieved. The latter is largely explained by the low profile of the Lotus valve, if proper size matching of the inner diameter of the surgical bioprosthesis is performed. In the current example, fluoroscopy demonstrated a small waist, indicating only slight oversizing of the 25 mm Lotus valve inside the 27 mm Hancock II surgical bioprosthesis.
Limitations
This is an observational study of only a single patient. Due to the pre-attached design of the Lotus valve, with its sophisticated engineering of the delivery catheter, only the transapical approach is currently feasible, providing the correct orientation for the valve. Last but not least, the pre-shaped long delivery catheter, with a rather rigid and curved catheter design, poses some challenges to the operators. Thus, delivery catheter modifications are desirable for the future.
Conclusion
In general, the case described here demonstrates the feasibility of TMViVI for treatment of a degenerated mitral bioprosthesis using a mechanically expanding THV. After TMViVI, there was complete resolution of mitral regurgitation and the patient had a satisfactory clinical outcome. Hence, TMViVI with the Lotus valve might become a valuable therapeutic alternative to balloon-expandable THV in patients deemed at prohibitive risk for redo open heart surgery.
| Impact on daily practice This intervention offers a safer approach to treat degenerated surgical mitral valves in high-risk surgical patients, since removal of the valve can be performed if an insufficient result has been obtained. In daily practice, single shot devices for this particular indication are likely to be replaced by resheathable devices in the future. Nevertheless, safety, feasibility and, in particular, durability need to be demonstrated. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. LV angiogram before TMViVI.
Moving image 2. Initial phase of Lotus valve implantation.
Moving image 3. Lotus valve implantation continued.
Moving image 4. Locking the Lotus valve.
Moving image 5. Assessment of locking function.
Moving image 6. Deployment of Lotus valve.
Moving image 7. TEE with colour Doppler after TMViVI.
Moving image 8. LV angiogram after TMViVI.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. LV angiogram before TMViVI.
Moving image 2. Initial phase of Lotus valve implantation.
Moving image 3. Lotus valve implantation continued.
Moving image 4. Locking the Lotus valve.
Moving image 5. Assessment of locking function.
Moving image 6. Deployment of Lotus valve.
Moving image 7. TEE with colour Doppler after TMViVI.
Moving image 8. LV angiogram after TMViVI.

