Abstract
Aims: Structural deterioration and paravalvular leak (PVL) are complications associated with surgically implanted prosthetic valves, historically requiring reoperation. We present our experience of complete transcatheter repair of a degenerated mitral bioprosthesis.
Methods and results: From March 2012 to October 2012, we reviewed consecutive, high-risk surgical patients (n=5) who underwent transcatheter repair of a failed mitral bioprosthesis with severe paravalvular regurgitation (PVR). Manufacturer valve sizes ranged from 27 to 33 mm, regurgitation (n=1), stenosis (n=1), or both (n=3). Percutaneous transapical and transseptal access were achieved with PVL closure performed transapically. An arteriovenous rail was created for transseptal delivery of a Melody valve. All patients had successful PVL closure with no residual PVR. Valve-in-valve (ViV) implantation was successful in four patients. Overall, mean transvalvular mitral gradient was 11.2 mmHg pre-procedure which improved to 5 mmHg post-procedure. Improvement of NYHA Class ≥2 was achieved in all patients (19±3 months). One patient had controlled Melody valve embolisation which required emergent surgical replacement. Inner valve diameter was 26 mm, too large for Melody valve implantation.
Conclusions: Complete transcatheter repair of a degenerated mitral bioprosthesis with PVR can be performed in the high-risk patient. Accurate measurement is necessary prior to intervention, with concern for embolisation among the larger valve sizes (>31 mm).
Abbreviations
AV: arteriovenous
CTA: computed tomographic angiography
LV: left ventricular:
PVL: paravalvular leak
PVR: paravalvular regurgitation
TA: transapical
TE: transoesophageal echocardiography
TTE: transthoracic echocardiography
ViV: valve-in-valve
Introduction
Surgical bioprosthetic heart valves have the advantage over mechanical valves of fewer thrombotic events and freedom from anticoagulation, at the expense of reduced durability and long-term structural deterioration1,2. In addition, paravalvular regurgitation (PVR), an uncommon yet serious complication, may occur with both biological and mechanical prostheses3. Early paravalvular leaks (PVLs) are usually associated with the technical aspects of the surgical implant, while late PVLs are commonly a consequence of suture dehiscence caused by gradual resorption of incompletely debrided annular calcification or bacterial endocarditis.
For both bioprosthetic valve degeneration and paravalvular regurgitation, the gold standard and only available treatment, until recently, has been surgery. Failure rates for surgical PVL repair can be as high as 35% with mortality rates that increase with each reintervention4. Percutaneous PVL closure has shown reasonable technical success and clinical outcomes without significant operative mortality5-7. In addition, transcatheter valve-in-valve (ViV) implantation for failing bioprosthetic valves has recently been utilised as an alternative to surgical valve replacement in high-risk or inoperable patients8. The purpose of this case series is to report our single-centre experience of complete repair of a failing mitral bioprosthesis and associated paravalvular leak using a transcatheter approach via percutaneous transseptal and transapical access.
Methods
This case series includes consecutive patients (n=5) who underwent complete transcatheter repair of a failed mitral bioprosthesis with paravalvular regurgitation at Lenox Hill Hospital Heart and Vascular Institute, North Shore/LIJ Health System, from March 2012 to October 2012. We chose a strategy of using a transapical (TA) approach for ease of access to mitral PVLs and for creation of an arteriovenous rail to promote coaxial valve positioning. Due to concern over difficulty accessing the PVL after ViV implantation and to avoid repeat TA puncture, both PVL closure and ViV implantation were performed in sequence during the same procedure.
The patients’ demographics are summarised in Table 1. Overall mean age was 73±12 years. All patients were deemed high risk for surgery with average STS score and logistic EuroSCORE of 15.1%±12 and 25.9%±15, respectively. Patients 3 and 4 had STS scores less than 10% but were deemed too high risk by two cardiothoracic surgeons due to multiple recent prior sternotomies/thoracotomies (Patient 3) and medical comorbidities (Marfans syndrome in Patient 4). All patients had congestive heart failure and three patients had haemolytic anaemia. One patient (Patient 4) had bioprostheses in both the mitral and aortic positions. Valve characteristics and mode of failure are identified in Table 2.
Patients were informed about the procedural risks, therapeutic alternatives, and the “off-label” use of both Amplatzer (St. Jude Medical, St. Paul, MN, USA) closure devices and the Melody valve (Medtronic, Minneapolis, MN, USA). During the initial study period, the SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) was not approved for use in the USA. When the SAPIEN valve was approved during the latter part of the study, it included only the Retroflex 3 (Edwards Lifesciences) delivery system with large outer diameters, the large delivery system being a concern for safe delivery via a percutaneous transseptal approach. Written informed consent was obtained prior to the procedure. The study was approved by North Shore/LIJ Health System’s Institutional Review Board for compassionate use and the FDA was notified.
Imaging
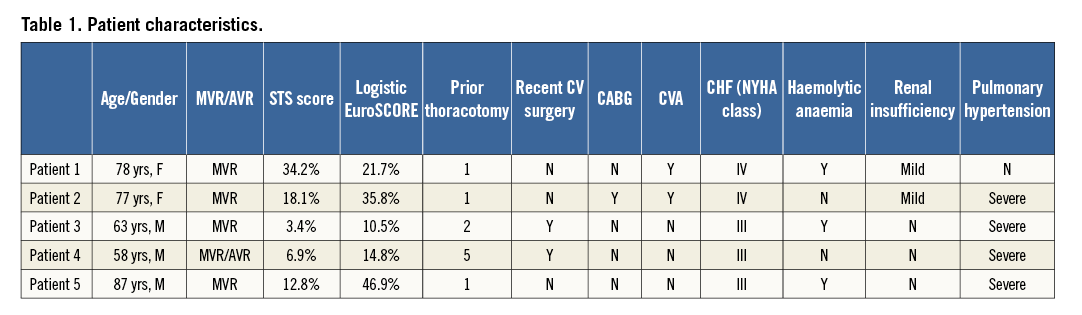
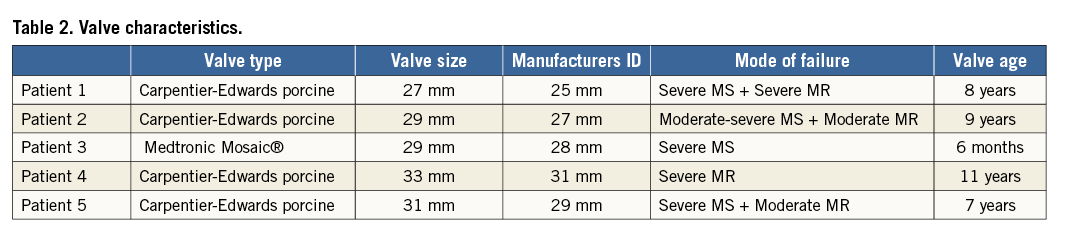
Each patient was evaluated by pre-procedural transthoracic (TTE) and transoesophageal (TEE) echocardiography, with TEE used for intraprocedural guidance. Three-dimensional (3D) imaging was performed using the IE33 system with an x7-2T probe (Philips Healthcare, Andover, MA, USA). Images were obtained using live 3D, 3D zoom, and full-volume modalities. The evaluation of severity of bioprosthetic dysfunction and PVR, along with PVL localisation and morphology assessment, were performed based on the American Society of Echocardiography’s guidelines9. Patients also underwent cardiac CTA (256-slice iCT scanner; Philips Healthcare, Cleveland, OH, USA) using helical scan mode with multiphase acquisition (16 phases, 6.25% RR interval increments) and retrospective ECG gating. Non-ionic contrast media injection of 60 to 90 ml at a rate of 5 to 6 ml/sec was utilised, depending on the patient size and renal function. The timing between contrast injection and the beginning of image acquisition was determined with the aim of peak contrast concentration in the left ventricle. Both pre- and post-procedural CTA were performed. Echocardiographic parameters, PVL location by surgical view and size, and bioprosthesis inner dimensions by both 3D TEE and CTA (including pannus/leaflet thickening) are reported in Table 3 and exemplified in Figure 1.
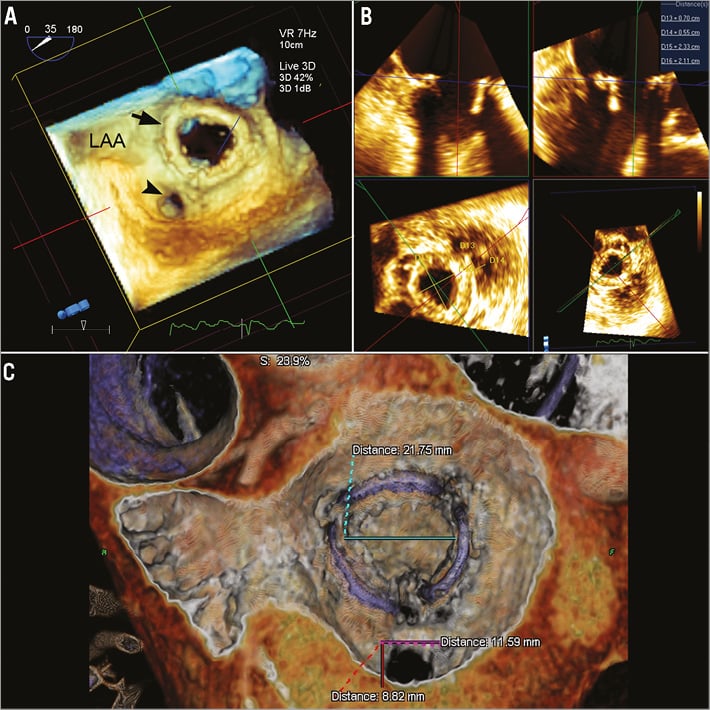
Figure 1. Multimodality imaging of paravalvular leaks. A) 3D transoesophageal echocardiography (TEE) reveals a large mitral paravalvular leak (PVL) at the 6 o’clock position in the surgical view from the left atrial perspective. LAA: left atrial appendage, black arrowhead: PVL, black arrow: mitral prosthesis. B) Measurements of length, width and area of the PVL and the inner diameter of the mitral prosthesis can be performed on 3D TEE using QLAB (Philips, Amsterdam, The Netherlands). C) Computed tomographic angiography with 3D/4D reconstruction (TeraRecon, Inc., Foster City, CA, USA) shows the same mitral PVL with additional measurements of PVL length and width and the inner diameter of the prosthesis including pannus and thickened leaflets.
Intraprocedural CTA-fluoroscopy fusion imaging was utilised using prototype proprietary software, HeartNavigator (Philips Healthcare, Best, The Netherlands). Pre-procedural two-dimensional (2D) CTA images were 3D volume-rendered and automatically segmented to identify the right and left atrium, right and left ventricle, and aorta. Segmentation of the coronary arteries, lungs, and ribs was performed manually. The images were then analysed and a transapical access (TA) route was determined, such that entry into the LV was aligned with the centre of the bioprosthesis and away from lung parenchyma and coronary arteries10,11.
The transseptal access route was also determined such that puncture was performed posterior and superior in the interatrial septum. Approximately 3 to 4 cm above the mitral valve was desired to allow for the delivery sheath to orient within the left atrium such that it was coaxial with the bioprosthesis and without sharp angles to prevent kinking. Landmarks were placed labelling planned transseptal puncture, transapical skin entry, left ventricular entry, PVL, and centre of mitral bioprosthesis (Figure 2A). The CT images were then co-registered and overlaid onto live fluoroscopy by using the prosthetic valve frame or contrast aortogram for Patient 3 with limited fluoroscopic valve markers. Volume-rendered 3D images of the heart were replaced with an outlined view of the prosthetic valve and landmarks displayed for guidance (Figure 2B). To guide TA puncture, a cylinder was created on the HeartNavigator in direct line with the TA skin entry, LV entry, and valve centre.
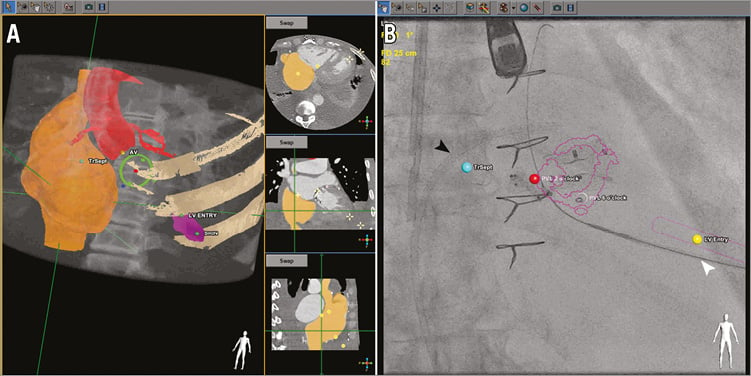
Figure 2. Pre-procedural planning and guidance using CT-fluoroscopy fusion imaging. A) Pre-procedural computed tomographic angiography (CTA) is volume-rendered and segmented (right atrium - orange, aorta with coronary arteries - red, mitral bioprosthesis frame - yellow). Landmarks are placed labelling skin entry (yellow dot), left ventricular entry (yellow dot), paravalvular leak (blue dot), centre of bioprosthesis (red dot), and cylinder (purple) identifying the direct line from skin entry to the bioprosthesis centre to guide transapical puncture. B) After registration of CTA with fluoroscopy, an outlined view of a stentless bioprosthesis and a cylinder marking transapical access can be identified (purple), along with landmarks for LV entry (yellow dot), transseptal access (blue dot), and the paravalvular leak (red dot). Transseptal sheath, black arrowhead; transapical sheath, white arrowhead.
Interventional technique
The procedure was performed under general anaesthesia. A 6 Fr sheath was inserted into the right common femoral artery and an 8 Fr sheath into the right common femoral vein. The 8 Fr sheath was upsized to a 24 Fr Gore® DrySeal sheath (Gore Medical, Flagstaff, AZ, USA). Transseptal and transapical puncture were performed under CTA-fluoroscopy fusion imaging and TEE guidance. Transseptal puncture was performed with adequate distance from the mitral bioprosthesis and an 8 Fr sheath (St. Jude Medical) was advanced into the left atrium. Transapical puncture was then performed using a 21 gauge Micropuncture® needle (Cook Medical, Bloomington, IN, USA) with contrast injection to confirm access into the left ventricle. After establishing LV entry, a 0.018-inch guidewire was advanced through the needle and exchanged for a 6 Fr 23 cm radial sheath (Cook Medical).
Heparin was administered to achieve an ACT >250 seconds. The mitral PVL was crossed using a hydrophilic glidewire from the transapical approach and the radial sheath advanced across the PVL into the left atrium. A safety wire, 0.018-inch Inoue, was placed into the left atrium and the sheath upsized to an appropriately sized delivery catheter to accommodate the selected Amplatzer occluder device(s) (St. Jude Medical) if necessary. A single device was placed in the PVL for Patients 1 and 2 and two devices were simultaneously placed for Patients 3 and 4 (Table 3, Figure 3A, Figure 3B). Patient 5 had two PVLs with each closed using a single occluder.
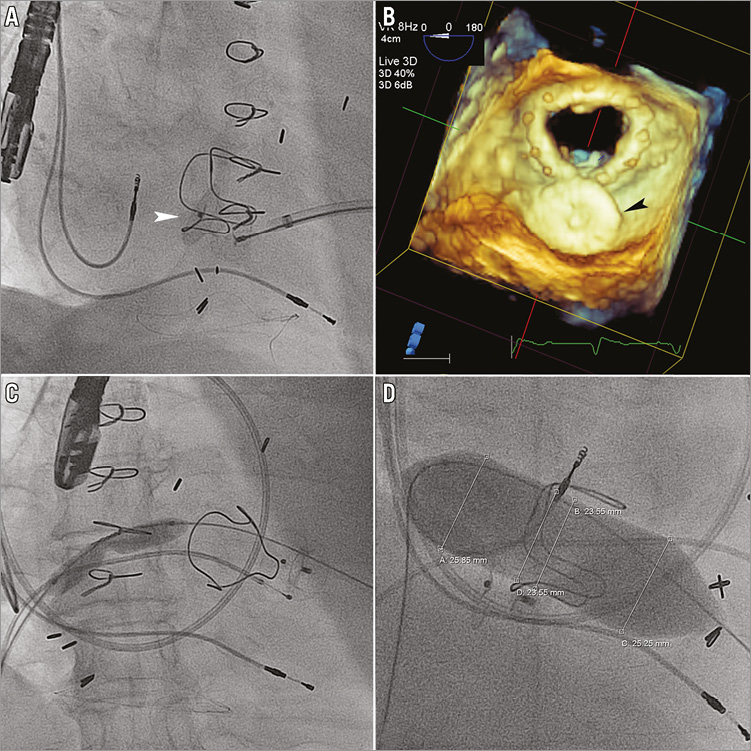
Figure 3. Initial procedural steps of complete transcatheter repair. A) Transapical closure of a mitral paravalvular leak (PVL) using an Amplatzer ductal occluder (white arrowhead: ADO; St. Jude Medical). B) 3D transoesophageal echocardiography from the left atrial perspective for procedural guidance shows the ADO (black arrowhead) completely occupying the PVL. C) & D) Balloon septostomy of the interatrial septum and balloon sizing of the mitral bioprosthesis are shown.
Next, a 0.035-inch glidewire was advanced from the transseptal sheath across the mitral bioprosthesis into the ascending aorta and exteriorised transapically using an 18-30 mm Ensnare (Merit Medical, South Jordan, UT, USA), creating an arteriovenous (AV) rail. Balloon septostomy was performed using an 8.0×40 mm peripheral balloon, inflated to nominal pressures (Figure 3C). The glidewire was exchanged for a 0.035-inch Amplatz Extra-Stiff wire (St. Jude Medical), by exteriorising a 110 cm 4 Fr Glide Catheter (Terumo Cardiovascular Systems, Ann Arbor, MI, USA). The 8 Fr transseptal sheath was then exchanged for a ZMED II 25×60 mm balloon (NuMED Inc., Hopkinton, NY, USA), inflated across the bioprosthetic mitral valve for inner diameter sizing (Figure 3D). The valve inner diameter was measured at a maximum of 23 mm for Patients 1-3 and 5, and 25 mm for Patient 4 (Table 4).
A 24 Fr Keller-Timmermans sheath (Cook Medical) was then advanced across the interatrial septum and mitral valve into the LV cavity with the TA sheath protecting the distal end of the dilator from injuring the LV myocardium (Figure 4A). Tension on the AV rail was adjusted to provide support for sheath delivery. The Melody valve was prepared per protocol on a 24 mm BiB balloon (NuMED Inc.) and advanced through the sheath. The valve was unsheathed with the sheath pulled back into the right atrium and the inner balloon inflated first to allow for positioning followed by final outer balloon inflation (Figure 4B, Figure 4C). The optimal angle for deployment was determined based on CTA and CTA-fluoroscopy fusion such that the fluoroscopy plane was perpendicular to the valve (Figure 4D). Fluoroscopy and TEE were used to confirm that the Melody valve was positioned with the valve proximal to the base ring of the bioprosthesis (Figure 4E, Figure 4F). The depth of valve implant was intended to be within the bioprosthesis struts to avoid LV outflow tract obstruction with a too ventricular or angulated implant.
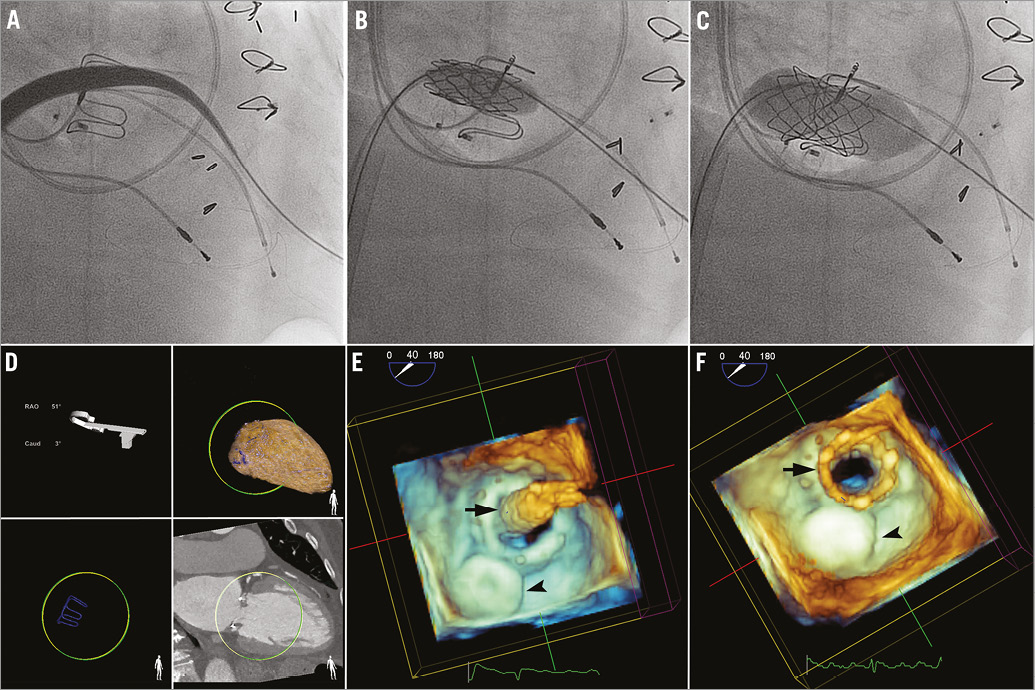
Figure 4. Percutaneous transseptal-transapical mitral valve-in-valve implantation. A) 24 Fr Keller-Timmermans sheath (Cook Medical) can be seen advanced across the interatrial septum and mitral bioprosthesis over the arteriovenous (AV) rail with the transapical sheath protecting the ventricular myocardium. B) The valve was unsheathed and inner balloon (BiB; NuMED Inc.) inflated to allow for accurate positioning. C) Final deployment performed with inflation of the outer balloon. D) Optimal deployment angle determined based on computed tomographic angiography (CTA) and CTA-fluoroscopy fusion imaging (HeartNavigator; Philips Healthcare). 3D transoesophageal echocardiography shows valve positioning with the inner balloon inflated (black arrow) (E) and final Melody ViV deployment (black arrow) with the AV rail remaining (F). Amplatzer ductal occluder, black arrowhead.
Transapical access was closed using a 6-4 mm Amplatzer ductal occluder (St. Jude Medical) and Surgiflo (Ethicon Inc., Somerville, NJ, USA) administered through the tract10. In Patients 4 and 5, significant shunting was noted across the interatrial septostomy site. Amplatzer Septal Occluders (St. Jude Medical), 20 mm and 14 mm respectively, were used to close the access site. Post-procedural CTA confirmed appropriate positioning of the implanted devices (Figure 5).
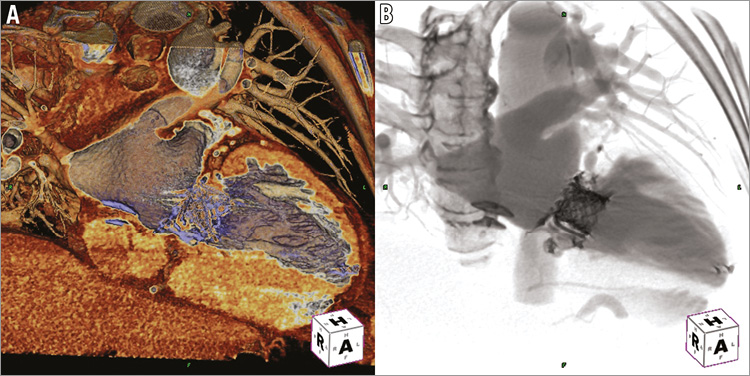
Figure 5. Post-procedural computed tomographic angiography. Computed tomographic angiography with 3D/4D reconstruction using specialised templates for cardiac tissue (A) and fluoro-like imaging (B) confirms the appropriate positioning of interatrial septal occluder, paravalvular leak closure device, Melody ViV, and transapical closure device (TeraRecon, Inc.).
Results
All patients had successful PVL closure with no evidence of residual PVR (Table 5). Melody ViV implantation was successful in four of five patients. No complications associated with transseptal access including cardiac perforation, aortic puncture, and pericardial effusion ± tamponade or transapical access including lung or coronary puncture, haemothorax, pericardial effusion ± tamponade, closure device embolisation/migration, and conversion to surgical closure occurred. 3D TEE confirmed an appropriately positioned Melody ViV with only mild transvalvular regurgitation noted for Patient 3. Overall, mean antegrade transmitral gradient was 11.2 mmHg pre-procedure which improved to 5 mmHg post-procedure. Patient 5, who had primarily bioprosthetic stenosis without evidence of transvalvular regurgitation and severe paravalvular regurgitation, had a pre-procedural mean gradient of 14 mmHg which improved to 2 mmHg post-procedure. An improvement of NYHA Class ≥2 was achieved in all patients at 19±3 months of follow-up.
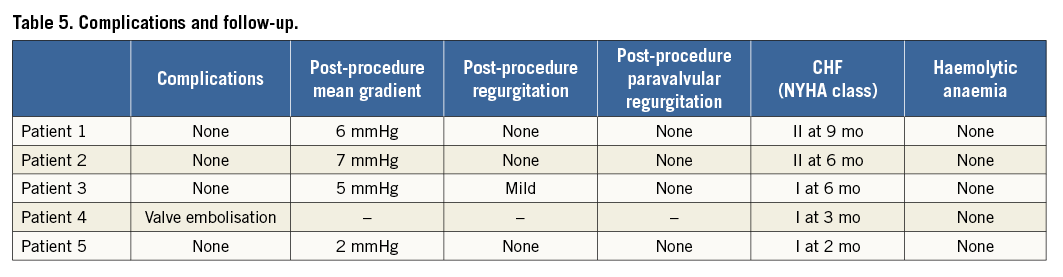
Patient 4 had controlled embolisation of the Melody valve. After delivery inflation and during removal of the BiB balloon, the valve was noted to have moved proximally towards the left atrium. An attempt was made to secure the valve in place using a second Melody valve (Melody ViV-in-valve) without success. Post-dilatation using a NuCLEUS-X 25×60 mm balloon (NuMED Inc.) failed to flare the distal ends of the Melody valves, in an attempt to lock the valves within the bioprosthesis. The decision was made to perform emergency surgical removal and replacement via a right thoracotomy approach. The valve was replaced with a 33 mm Carpentier-Edwards porcine valve (Edwards Lifesciences) without complication. The patient was discharged home on day five.
Upon re-review of the imaging and balloon sizing for Patient 4, the inner surgical valve diameter of the Carpentier-Edwards 33 mm valve was between 25 mm (CTA with pannus and balloon sizing) and 26 mm (TEE). Balloon sizing revealed similar sizing with a trivial waist identified at maximal balloon inflation. The measured inner dimensions on the surgically explanted valve confirmed such sizing. We now consider these measurements to be too large for Melody ViV implantation with this method. Since the maximal manufacturer recommended expansion is 24 mm and successful expansion to 25 mm has been reported, we do not recommend the described technique with inner dimensions >25 mm.
Discussion
The application of transcatheter therapy for high-risk and inoperable patients has recently been expanded in an effort to address the challenges of bioprosthetic valve failure, namely high morbidity and mortality rates from reoperation in patients with bioprosthetic dysfunction and PVL8,12-15. The first reported cases of a transcatheter valve in a failed surgical mitral bioprosthesis (ViV) were attempted through a percutaneous transseptal approach or direct transatrial approach via a right thoracotomy13. These approaches were technically challenging with unsuccessful deployment and embolisation occurring because of non-coaxial and too ventricular positioning of the prosthesis and lack of stable cannulation.
The surgical transapical approach has since been successfully performed and is now favoured because of its direct access and short distance anatomically to the mitral position and ease of manoeuvrability14,16. The technique of transapical mitral ViV implantation is similar to that of transapical transcatheter aortic valve replacement. It requires general anaesthesia and surgical exposure via a minithoracotomy through the 5th or 6th intercostal space. Nonetheless, there are significant risks associated with this approach, including major VARC-2 (Valve Academic Research Consortium) bleeding and stroke17-19.
The technique of transseptal-transapical approach described in this case series is completely percutaneous and is accomplished by utilising an AV rail. This approach allows for venous delivery of the transcatheter valve and the necessary coaxial positioning within the bioprosthesis, by adjusting tension on both sides of the rail. The location of the transapical sheath secures the position of the valve distally to prevent too ventricular positioning. In addition, it protects the LV from perforation by the delivery system. This approach minimises the necessary LV sheath size to only 6 Fr, when compared to a surgical transapical approach. Moreover, the balloon-in-balloon delivery system enables fine adjustments to facilitate accurate placement for final positioning and anchorage.
Successful mitral ViV implantation is highly dependent on proper positioning within the bioprosthesis as well as appropriate sizing13. It is important that the balloon-expandable transcatheter valves are as coaxial as possible within the failed prosthesis at the time of deployment and that angiographic imaging is in a plane perpendicular to the prosthesis to minimise foreshortening. Pannus, leaflet thickening and calcification may impede valve crossing and may limit full valve expansion, resulting in paravalvular or transvalvular regurgitation and increasing the likelihood of embolisation. Reduced durability of these valves also occurs when they are underexpanded. Sizing of a failed surgical bioprosthesis is crucial, and there are widespread differences in labelled and true dimensions among different manufacturers and models. 3D TEE, CTA, and balloon sizing can all confirm the inner valve diameter and ensure appropriate sizing for safe ViV seating. However, due to concerns of debris embolisation and acute mitral regurgitation from balloon sizing, we no longer utilise this technique unless the bioprosthesis is severely stenosed and impairs crossing or if it is not entirely apparent from TEE and CTA that the internal diameter would support the transcatheter valve.
Melody ViV implantation using antegrade transseptal-surgical transapical and percutaneous transseptal-transapical approaches have recently been described20-22. Furthermore, placement of a Melody ViV within a mitral annuloplasty ring in an animal model has been performed via percutaneous transfemoral venous and transatrial approaches23. Despite successful implantation, the long-term function of this valve when subjected to systemic pressure loads is still unknown. The Melody valve is that of a bovine jugular valved vein which is sutured into a platinum-iridium stent. It is designed for dysfunctional right ventricular outflow tract conduits. The reported results of seven Melody ViVs within a high-pressure haemodynamic environment (all aortic except for one mitral) revealed complete freedom from regurgitation and an 86% freedom from significant stenosis at one-year follow-up24. This series revealed functional Melody ViVs up to 22 months after implantation.
Alternative transcatheter valves, such as the SAPIEN valve which is designed for the aortic position, have been tested under left-sided loading conditions. Five-year follow-up data have demonstrated favourable results with excellent haemodynamic profiles and low rates of prosthetic valve failure25. Nonetheless, the long-term durability of transcatheter valves overall and their modes of failure remain unclear especially within surgically implanted bioprostheses.
The Melody valve was utilised in this case series for a multitude of reasons. The length of the Melody when compared to the SAPIEN valve is 23 mm to 26.2 mm (18 mm balloon, 26.2 mm; 20 mm balloon, 24.2 mm; 22 mm balloon, 23 mm) and 14.3 mm to 19.1 mm (23 mm balloon, 14.3 mm; 26 mm balloon, 16.1 mm), respectively, depending on final deployment size. This allows for ease of positioning (larger landing zone/margin of error) within the bioprosthesis, particularly important for prosthetic valves with limited fluoroscopic markers. Additionally, it is delivered via a lower profile delivery system (Melody: 24 Fr, SAPIEN: 25 Fr and 28 Fr) and was the only commercially valve available in the USA during our early experience to utilise in an off-label fashion.
In addition, the combined approach with percutaneous transapical access provided the opportunity to close mitral paravalvular leak(s). From previously published experience, the technical and clinical success of percutaneous PVL closure can be as high as 86% and 77%, respectively5-7. Increased success has been reported with utilisation of the transapical approach given its ease of access to all components of the mitral apparatus, including septally located mitral PVLs10,26. Early data for percutaneous closure suggest similar survival when compared to reported surgical results, with the worst outcomes in patients treated with conservative medical management3,7,27. Although technically challenging, the addition of percutaneous PVL closure to mitral ViV implantation in patients with combined bioprosthesis dysfunction and paravalvular regurgitation provides more complete, symptomatic treatment without the significant operative mortality associated with re-operation.
Our series of patients illustrates the ability to perform successfully complete transcatheter repair of a failing mitral bioprosthesis with paravalvular leak(s). Patients underwent both percutaneous PVL closure and Melody ViV implantation in the mitral position via percutaneous transseptal-transapical approaches. Though implantation of a transcatheter valve in the mitral position has limitations, we were able to hurdle many difficulties through percutaneous transapical access with creation of an AV rail and CT/fluoroscopy fusion imaging for guidance. The described percutaneous combination technique is a novelty that has never been reported, to our knowledge, in the literature. With improved transcatheter valve technologies and increasing long-term experience, this technique could play a significant role in the way we approach transcatheter-based valve repair in the mitral and perhaps in other positions.
Conclusions
Complete transcatheter repair of a degenerative surgical mitral bioprosthesis with significant paravalvular regurgitation can be performed in the high-risk patient while utilising combined transseptal and transapical approaches. Multimodality imaging that includes 3D TTE, 3D CTA, and CTA-fluoroscopy fusion imaging is required for detailed planning and procedural guidance. In addition, accurate measurement of the inner surgical valve dimension is critical prior to planned intervention, with concern for embolisation among the larger surgical prostheses (>31 mm). Further study is required to evaluate the safety and long-term durability of percutaneous implantation of the Melody ViV in the mitral position.
| Impact on daily practice Complications of bioprosthetic valves include leaflet degeneration and paravaluvlar leak(s). Many symptomatic patients are too high-risk or inoperable for surgical repair or replacement and given no therapeutic option. In patients with surgical valve sizes amenable to transcatheter ViV and without evidence of infective endocarditis or valve instability, complete percutaneous repair now provides an alternative treatment approach in this complex patient population. |
Conflict of interest statement
C. Kliger has received a speaking honorarium from St. Jude Medical. G. P. Fontana is a consultant for St. Jude Medical, Medtronic, Sorin Medical, Entourage Medical Technologies, Edwards Lifesciences, Paieon Inc., has received speaking honoraria from St. Jude Medical, Medtronic, Edwards Lifesciences, and Sorin Medical, and has stock/stock options in Entourage Medical Technologies. I. Kronzon is a consultant for Philips Healthcare. C. E. Ruiz is a consultant for Valtech, St. Jude Medical, Sorin Medical, receives research grants from Philips Healthcare, and has financial interest in Vascular Therapies, MitrAssist, Entourage, and BioInspire. The other authors have no conflicts of interest to declare.

