Abstract
As a result of recent randomised controlled trials and registry observations, transcatheter aortic valve replacement (TAVR) enjoys growing appeal for the treatment of patients at high or extreme risk from surgical aortic valve replacement. However, the current technologies and techniques have important limitations, including risk of stroke, vascular complications and paravalvular aortic regurgitation, which may in turn influence survival. While careful patient selection and screening may improve outcomes, new valve designs and iterations are required. The Lotus™ aortic valve replacement system is a new fully repositionable device designed to facilitate more precise delivery and minimise paravalvular regurgitation. The safety and efficacy of the Lotus valve are being studied systematically in the REPRISE clinical trial programme.
Introduction
Transcatheter aortic valve replacement (TAVR) has experienced rapid growth and increasing adoption since its clinical debut 10 years ago1. Although much of the evidence supporting its use in high or extreme risk patients with severe symptomatic aortic stenosis comes from clinical registries, randomised control trials have demonstrated very promising outcomes. When compared with conventional medical therapy, patients unsuitable for surgical valve replacement randomised to TAVR have a significant reduction in mortality and need for repeat hospitalisation at two years2, and high-risk patients suitable for either TAVR or surgical valve replacement have a similar risk of mortality3. Despite these landmark observations, the risks of stroke and paravalvular regurgitation following TAVR remain a concern3-5.
While greater understanding of the influence of patient comorbidities, aortic valve morphometry and geometry and careful patient selection will potentially enhance the role and benefit of TAVR6,7, improvements in valve design may reduce the risk of adverse events by reducing the learning curve, allowing more accurate placement, and minimising or eliminating paravalvular aortic regurgitation.
Device overview
The Lotus™ Valve System (Boston Scientific, Natick, MA, USA) has several features to facilitate safe and accurate device placement using an intuitive design with a high degree of user control. The Lotus Valve System comprises a bioprosthetic aortic valve implant and a catheter-based delivery system for introduction and delivery of the valve implant. To simplify handling, preparation and loading, the valve is pre-attached to the delivery system (Figure 1), eliminating the need for special tools or fixtures. The delivery handle incorporates a simple, ergonomic design that enables a controlled, predictable and accurate deployment process via two controls: a control knob and a release collar (Figure 2). The valve is smoothly and progressively unsheathed, deployed and locked with counterclockwise rotation of the control knob. If the initial deployment is suboptimal, the device can be subtly advanced or retracted as needed or even completely retracted into the delivery sheath at any time prior to the final release. Furthermore, the device can be safely retrieved during the procedure if the decision is made not to implant. The valve is released by sliding the release collar forward and turning the release mechanism. Images on the delivery handle indicate valve functions and turning directions. The valve functions early in deployment, providing haemodynamic stability for the patient and enabling the operator to complete the delivery process in a controlled and considered fashion.

Figure 1. Valve and delivery system.
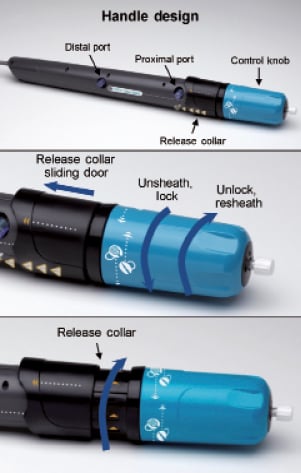
Figure 2. Delivery system handle design. The handle consists of two components: the control knob (blue), which unsheathes and locks the valve when rotated counterclockwise, or unlocks and resheathes the valve when rotated clockwise, and the release collar, which releases the valve from the delivery system with clockwise rotation. The handle is designed to ensure that the sheathing, locking and release steps are performed in the appropriate sequence. Reprinted from Cardiac Interventions Today10.
The bioprosthetic aortic valve implant comprises three bovine pericardial leaflets supported on a braided nitinol frame (Figure 3). A radiopaque marker is located at the vertical centre of the implant to aid positioning. An adaptive seal surrounding the ventricular portion of the device is designed to conform to irregular surfaces of the native anatomy, potentially reducing paravalvular aortic regurgitation.
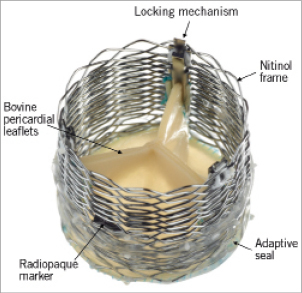
Figure 3. Lotus aortic valve components. The valve consists of three bovine pericardial leaflets attached to a braided nitinol frame. A radiopaque marker, situated in the centre of the valve frame, facilitates accurate placement, while the adaptive seal, located on the outside bottom half of the frame, is designed to minimise paravalvular regurgitation. The valve implant is attached to the delivery catheter through a locking mechanism. Reprinted from Cardiac Interventions Today10.
A clinical case is briefly reviewed here in order to provide insight into the Lotus valve delivery, deployment and final result. The first patient treated in the recently completed REPRISE I trial was an 82-year-old female with a body mass index of 16 (height 166 cm and weight of 44 kg), hypertension, hyperlipidaemia and a number of comorbidities, including asthma, scleroderma, and a history of right hip and femoral fracture. She presented with symptomatic aortic stenosis (New York Heart Association Class II). Echocardiography revealed a heavily calcified trileaflet aortic valve with an estimated peak and mean pressure gradient of 92 mmHg and 55 mmHg, respectively, an aortic valve area of 0.5 cm2, and a left ventricular ejection fraction of 65%. An angiogram prior to the procedure showed no significant coronary artery disease. Society of Thoracic Surgeons mortality and morbidity scores were calculated to be 4.1% and 19.2%, respectively, and the logistic EuroSCORE was 9.1%. Computed tomography measurements of the aortic root (annulus diameter and perimeter) as well as the iliofemoral structure confirmed anatomical fit for the 23 mm Lotus valve and its transfemoral delivery system and the Lotus introducer sheath.
In accordance with the REPRISE I protocol, the procedure was undertaken under general anaesthetic to facilitate continuous transoesophageal imaging. A temporary pacing wire was placed via a 6 Fr sheath in the right internal jugular vein and a 6 Fr sheath was placed in the left femoral artery. The right femoral artery was punctured under direct fluoroscopic and angiographic guidance aided by an internal mammary catheter inserted from the right femoral artery. Following a standard preclose technique, an 18 Fr Lotus introducer sheath was passed through the right femoral and iliac arterial system under continuous fluoroscopic visualisation and then positioned in the descending aorta. The aortic valve was crossed using conventional techniques and a super stiff guidewire (0.035”, 260 cm) was positioned carefully in the left ventricle before balloon valvuloplasty was performed with an 18 mm by 40 mm balloon during rapid ventricular pacing. There was no significant waist following valvuloplasty. Images and a detailed description of the Lotus valve implantation procedure are shown in Figure 4. Throughout the procedure the patient’s blood pressure remained stable. The Lotus valve was successfully positioned and implanted on the first attempt and no repositioning was needed. The patient was discharged from the hospital five days post procedure. No aortic regurgitation was present either on the discharge or on the 30-day echocardiogram. Echocardiographic assessments revealed a mean gradient of 13.2 mmHg and an aortic valve area of 1.6 cm2 at discharge; values at 30 days post procedure were 16.5 mmHg and 1.6 cm2, respectively.
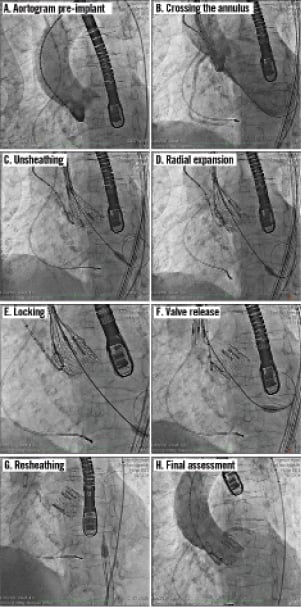
Figure 4. Sequential angiographic images of the Lotus valve deployment, positioning, locking and release. A) Pre-implant aortogram; B) Advancement across the annulus – tip of the catheter just below the basal plane (annulus); C) Unsheathing – initiated by rotating the control knob of the delivery handle counterclockwise. During unsheathing, the initially elongated valve frame shortens and radially expands. The valve is positioned in the aortic annulus by maintaining the radiopaque marker a few millimetres above the basal plane, while the valve is being fully expanded; D) Radial expansion – as unsheathing continues, the valve expands radially and anchors within the aortic annulus; E) Valve locking; F) Valve release – valve release is accomplished by rotating the release collar clockwise, resulting in the release of the valve from the delivery catheter; G) Resheathing – once the valve is released, the delivery catheter is resheathed and the nose cone reseated, and the device is retracted through the introducer sheath; H) Final assessment
REPRISE clinical programme
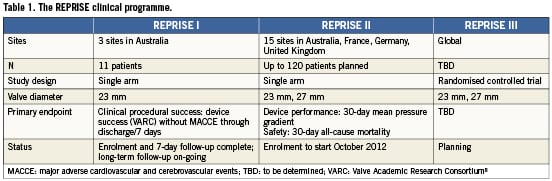
The Lotus valve is being evaluated in the REPRISE clinical programme. The characteristics of the three planned clinical studies are outlined in Table 1. Enrolment in the REPRISE I feasibility study is complete. REPRISE II is expected to begin enrolment in October 2012, and REPRISE III is currently in the planning stages.
REPRISE I is a prospective, single-arm, three-centre feasibility study designed to assess acute safety and performance of the 23 mm Lotus valve in symptomatic patients with calcified stenotic aortic valves who are considered high risk for surgical valve replacement. The primary endpoint was clinical procedural success, defined as successful implantation of a Lotus Valve System (device success) without in-hospital major adverse cardiovascular and cerebrovascular events (MACCE) through to discharge or seven days post procedure, whichever came first. Device success and MACCE were defined in accordance with the Valve Academic Research Consortium (VARC) definitions8. Secondary endpoints included successful repositioning of the Lotus Valve System (if attempted), successful retrieval of the Lotus Valve System (if attempted), and the incidence of central and paravalvular aortic valve regurgitation. Other key endpoints include bleeding, acute kidney injury, vascular complication rates, new conduction disturbances, cardiac arrhythmias and the indications for new permanent pacemakers. Parameters of valve performance, such as effective orifice area, mean and peak aortic gradients, peak aortic velocity, the severity of aortic regurgitation, and other metrics of cardiac function, will be assessed by sequential, core-laboratory reviewed transthoracic echocardiography studies. Functional status will be evaluated by the New York Heart Association Class, and other indices of health status and quality of life will be assessed using the SF-12 and EQ-5D questionnaires at baseline, six months, and one year post valve implantation.
As of May 2012, REPRISE I enrolment and seven-day follow-up was complete with 11 patients enrolled at three centres in Australia (Monash Medical Center, Melbourne; Royal Adelaide, Adelaide; St. Vincent’s Hospital, Melbourne). Additional follow-up is planned at 30 days, three months, six months, 12 months, and annually to five years post index procedure. Primary endpoint results were presented at the Paris Course on Revascularization in Paris, France (May 15-18, 2012)9 and preparation of the primary endpoint manuscript is underway.
Summary
The Lotus aortic valve replacement system is a fully repositionable device designed to facilitate accurate delivery and minimise paravalvular regurgitation. The valve can be positioned precisely and successfully with little or no aortic regurgitation post procedure, as highlighted in the clinical case. Results of the REPRISE clinical programme will provide safety and efficacy outcomes on the use of this device in appropriately selected patients with symptomatic and severe calcific aortic stenosis.
Of note, 2012 coincided with the five-year follow-up of the first patient implanted with the first-generation Lotus Valve System. The patient was 98 years old at the time of the five-year visit and completely mobile, independent and in good health, and had no symptoms of heart failure. Site-reported transthoracic echocardiographic images (mean pressure gradient 16 mmHg and aortic valve area 1.8 cm2) demonstrated normal functioning of the Lotus valve at five years. Continued follow-up will provide additional data on the long-term clinical outcomes and durability of the Lotus valve.
Acknowledgements
The authors thank Blessie Concepcion of Boston Scientific for support of the case study and the REPRISE clinical programme.
Conflict of interest statement
I.T. Meredith serves on the scientific advisory boards for and receives honoraria from Boston Scientific. K.L. Hood, D.J. Allocco, N. Haratani and K.D. Dawkins are full-time employees with equity interest in Boston Scientific. The Lotus™ Valve System is an investigational device manufactured by Boston Scientific.

