Introduction
First-in-human clinical studies and in vitro tests for valve-in-valve (ViV) with the ALLEGRA (New Valve Technology) transcatheter heart valve (THV), as well as the 30-day data of the VIVALL study demonstrated favourable results, even in small surgical aortic bioprostheses123. This paper reports the 12-month outcomes of the VIVALL study.
Methods
STUDY DESIGN AND POPULATION
The prospective, multicentre, single-arm VIVALL study (clinicaltrials.gov: NCT03287856) investigated the technical feasibility of treating failing surgical aortic valves (SAV) with the ALLEGRA THV. Details on the design of the VIVALL study and 30-day results have been published previously3. All data were adjudicated by an independent combined data safety monitoring (DSM)-clinical events committee (CEC) and core lab. Thirty symptomatic patients (mean age 78.6±6.0 years, 50.0% female, Society of Thoracic Surgeons [STS] score 4.5±2.1%) with a failing SAV were treated and followed up at 30 days, 6 months and 12 months. Patient demographics have been reported previously and are detailed in Supplementary Table 13.
STATISTICAL ANALYSIS
Descriptive statistics and p-values of all metrics were calculated for each visit and defined changes relative to pre-treatment and Kaplan-Meier curves were calculated.
Results
FOLLOW-UP
Overall, 28, 25 and 24 patients completed the 30-day, 6-month and 12-month visits, respectively. One patient who received a second transcatheter aortic valve implantation (TAVI) was followed up for safety only. Another patient refused to return to the hospital after 30 days and 2 patients missed the 6-month and 12-month visits (Supplementary Figure 1).
CLINICAL RESULTS
Consistent improvements in functional status after implantation of the ALLEGRA THV were observed. At baseline, 79.3% of the patients were in New York Heart Association (NYHA) Class III and IV. After one year, 83.2% of the patients were in NYHA Class I and II and only 16.7% remained in NYHA Class III (Supplementary Figure 2).
There were 3 deaths between 30 days and 12 months (Supplementary Figure 3), leading to survival rates of 93.3% at 6 months and 90% at 12 months. The causes of death were pneumonia (106 days post-procedure), prostate carcinoma (118 days post-procedure) and a fatal road accident (237 days post-procedure). Cardiovascular mortality was 0% at all follow-ups. No disabling/non-disabling strokes, life-threatening bleedings, acute kidney injuries (AKI stage 2 and 3), coronary artery obstructions requiring intervention, structural valve deteriorations, valve-related dysfunctions, prosthetic valve endocarditis or myocardial infarctions were reported up to 12 months (Central illustration). The need for de novo pacemaker implantation was 3.8% at 12-month follow-up (Table 1).
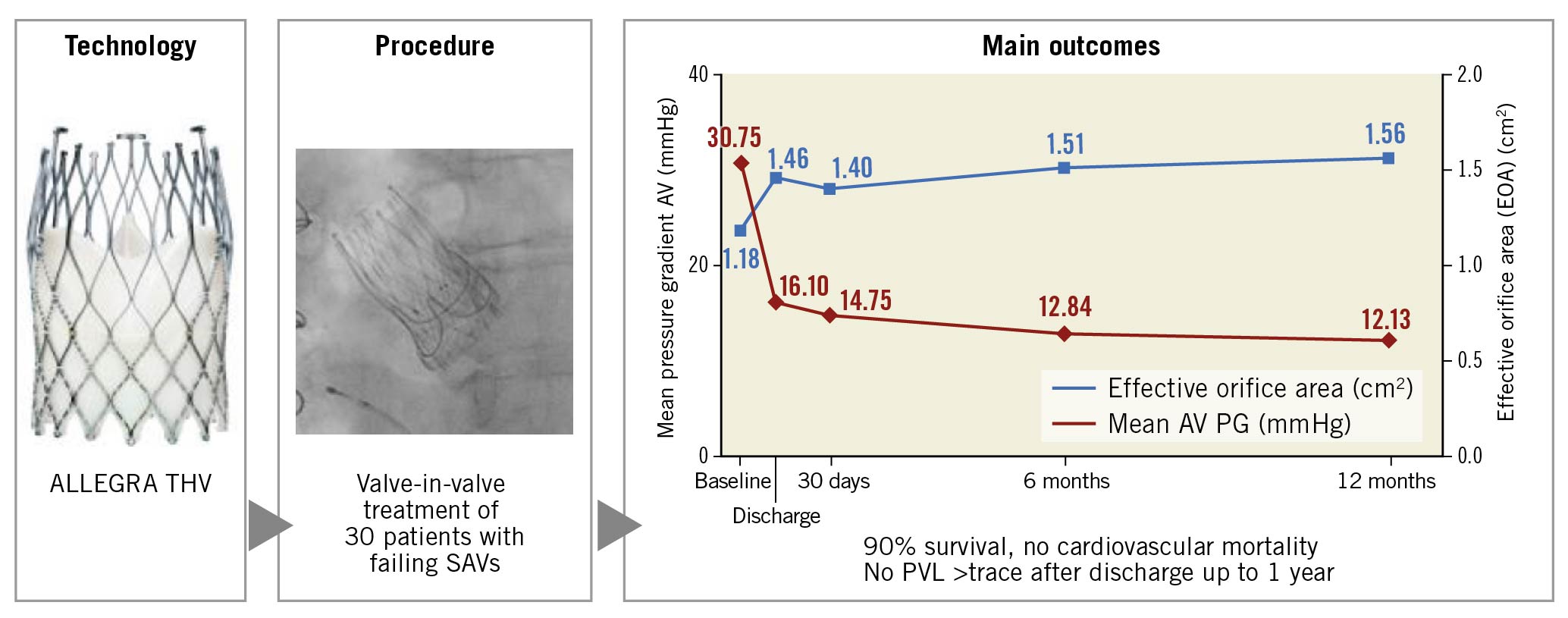
Central illustration. Treatment of degenerated surgical valves with the ALLEGRA THV (VIVALL study): one-year outcome.
ECHOCARDIOGRAPHIC HAEMODYNAMIC OUTCOMES
The mean gradient across the aortic valve consistently decreased at 6 months and 12 months, with a corresponding increase in effective orifice area (EOA) (Figure 1). A haemodynamic subanalysis according to the failure mode of the bioprostheses (Figure 2A, Figure 2B) as well as the depth of implantation (implantation depth – mixed failure: 3.5±1.8 mm, stenosis: 3.9±2.3 mm; insufficiency: 6.4±2.8 mm) revealed no significant differences between groups (data not shown). No patients developed mild, moderate or severe paravalvular leak (PVL) from discharge until 12 months.
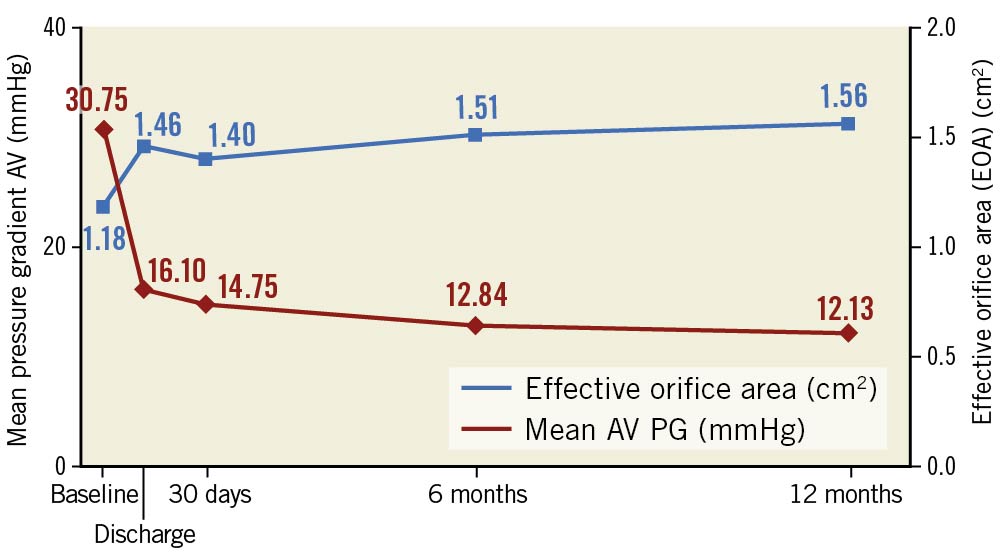
Figure 1. Echocardiographic haemodynamic data.
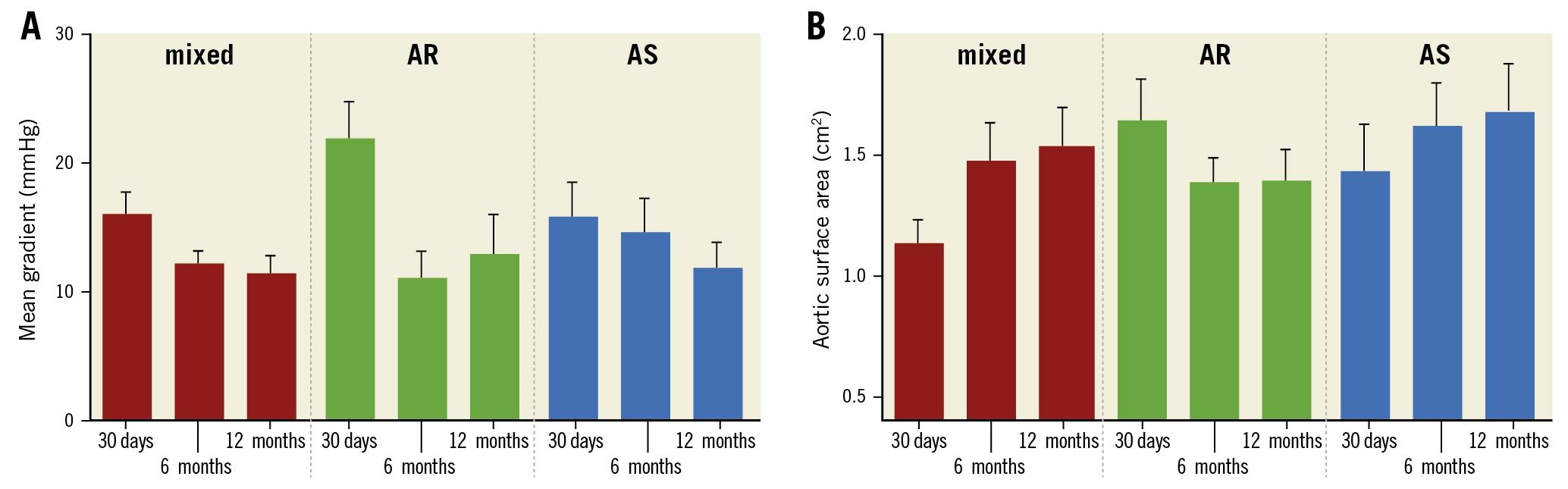
Figure 2. Subanalysis of echocardiographic haemodynamic data (A: mean gradient, B: aortic surface area) according to bioprosthetic failure mode.
Discussion
The VIVALL study confirmed the safety and performance of the ALLEGRA THV up to one year, with a high survival rate of 90% and progressive improvement in haemodynamics and functional capacity (Figure 1). No relationship between haemodynamics and implantation depth or failure mode of the treated SAV was observed. In comparison to the recently published VIVA trial4 the observed haemodynamics were similarly improved, despite a more challenging patient cohort with a higher rate of small bioprostheses in VIVALL (Mitroflow, 43%; Mosaic®, 6.7%).
Limitations
The VIVALL study was limited by the single-arm design and the low patient numbers. Hence, the sub-analysis can only be taken as exploratory.
Conclusion
The use of the ALLEGRA THV is safe and effective in the ViV treatment of failing SAVs, even with very small inner diameters.
Impact on daily practice
Many elderly patients with failing surgical bioprostheses have an increased risk for surgical reoperation. Thus, TAVI systems with proven safety results and favourable haemodynamics, such as the ALLEGRA THV, provide a promising treatment alternative.
Funding
This study was funded by NVT GmbH.
Conflict of interest statement
U. Schäfer reports being a consultant, a proctor and on the speaker’s bureau for Abbott Vascular, Boston Scientific, Edwards Lifesciences, Medtronic, Gore, Jena Valve Technology, and New Valve Technology, and receiving research support from Abbott Vascular, Boston Scientific, Jena Valve Technology, Edwards Lifesciences and New Valve Technology. C. Butter reports being on the speaker’s bureau for Edwards Lifesciences and Medtronic, and receiving research support from New Valve Technology. M. Landt reports research support from New Valve Technology. C. Frerker reports being on the speaker’s bureau for and receiving travel grants from Abbott Vascular, Boston Scientific, Edwards Lifesciences and Medtronic, and receiving research support from New Valve Technology. H. Treede reports being a consultant for and being on the speaker’s bureau for Abbott Vascular, Boston Scientific, Edwards Lifesciences, Jena Valve Technology, and Medtronic, and receiving research support from Abbott Vascular, Boston Scientific, Edwards Lifesciences, Jena Valve Technology and New Valve Technology. J. Schirmer reports being a proctor for Boston Scientific and Jena Valve Technology, being on the speaker’s bureau for and receiving travel grants from Edwards Lifesciences and Medtronic, and receiving grants from New Valve Technology. C. Koban reports receiving research support from New Valve Technology. A. Allali reports receiving research support from New Valve Technology. T. Schmidt reports being on the speaker’s bureau of and receiving travel grants from Edwards Lifesciences, Boston Scientific and Medtronic. E. Charitos reports receiving research support from New Valve Technology. L. Conradi reports being a consultant for Edwards Lifesciences, Boston Scientific, Abbott Vascular, and Jena Valve Technology, being a speaker for Edwards Lifesciences, Boston Scientific, Abbott Vascular, Medtronic and Jena Valve Technology, and receiving research support from Abbott Vascular, Boston Scientific, Jena Valve Technology, Edwards Lifesciences and New Valve Technology. O. Nikolayevska has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

