
Since Alain Cribier performed the first compassionate intervention in April 2002, the SAPIEN valve has undergone continuous improvements which were incorporated into the design of the SAPIEN XT, SAPIEN 3 (S3) and more recently the SAPIEN 3 Ultra (all Edwards Lifesciences, Irvine, CA, USA).
The development of the SAPIEN 3 Ultra (fifth-generation balloon-expandable valve) was initiated several years ago. The objective was to simplify the procedure by using a new version of the eSheath™ (Axela; Edwards Lifesciences), a delivery system with an enhanced profile, and an on-balloon valve crimping system in order to obviate the need for positioning the valve on the balloon in the descending aorta. The design of the valve’s metallic structure has been slightly modified, but the height of the deployed valve is the same as that of the S3; the outer skirt is 40% higher compared to the S3 and has greater absorption properties in order to reduce the risk of paravalvular leak (PVL) further (Figure 1).
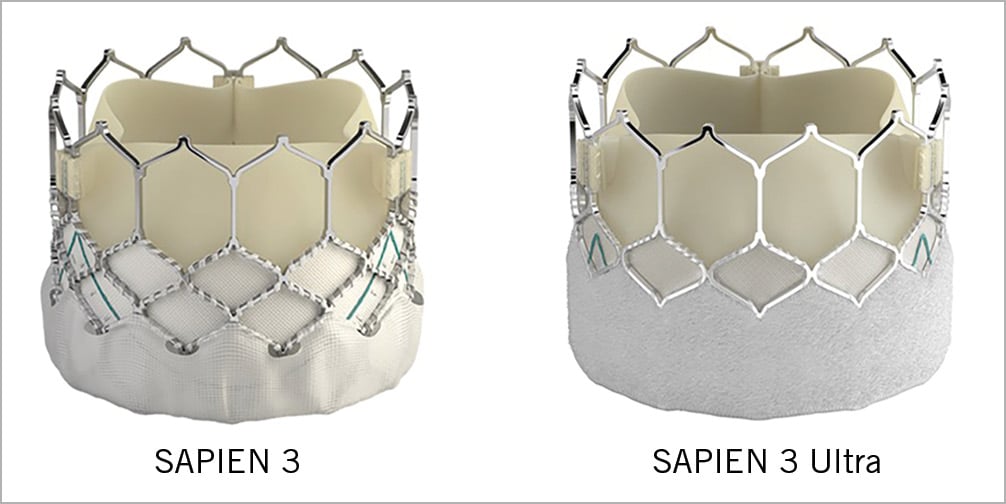
Figure 1. Comparison between the SAPIEN 3 and the SAPIEN 3 Ultra transcatheter heart valves.
During the recently held PCR London Valves meeting, John Webb presented the first results achieved with the SAPIEN 3 Ultra in a prospective, multicentre study conducted in Canada and the UK which included 83 relatively selected intermediate-risk patients with calcific aortic stenosis in a tricuspid valve, a mean age of 83 and an STS score of 3.6 (Webb J. A prospective multicentre study of the SAPIEN 3 Ultra system in intermediate-risk patients with severe aortic stenosis. Presented at PCR London Valves, London, 18 November 2019). Procedural success (freedom from mortality, conversion to surgery and moderate to severe PVL) was achieved in 100% of cases with 0% moderate to severe PVL and single-digit new pacemaker implantation at 30 days.
In this issue of EuroIntervention, Saia et al1 present in turn the results of a European, multicentre, prospective medium-sized study conducted in 130 all-comers (mean age of 81, logistic EuroSCORE of 14% and an STS score of 3.8) who were treated with the SAPIEN 3 Ultra valve (20, 23, 26 mm) and the SAPIEN 3 (29 mm), the Axela eSheath and the Ultra delivery system. The SAPIEN 3 Ultra obtained CE marking in November 2018; patients were included consecutively in selected centres from November 2018 to February 2019.
Notwithstanding the intrinsic limitations of an observational study with unavoidable inclusion and operator bias on account of previous experience, the 30-day results are as encouraging as those presented by John Webb, namely, 100% implantation success, no stroke, no myocardial infarction, 2.2% rate of major vascular complications, 1.4% >grade I PVL and, above all, an exceptionally low rate of pacemaker implantation which was required in only 4.4% of patients at 30 days.
The SAPIEN 3 Ultra was launched on 3 January 2019 in the USA. In July 2019, Edwards Lifesciences sent an urgent field safety notice to customers, warning them that they had received reports of burst balloons during implantation procedures and insisting on the usual recommendations for safe valve deployment. In August 2019, the FDA recalled the SAPIEN 3 Ultra delivery system and issued similar recommendations for optimal use of the Ultra valve.
Since the FDA recall and based on feedback from the valve’s users, the SAPIEN 3 Ultra is now distributed with the valve pre-mounted on the classic Commander delivery system (Edwards Lifesciences) and the eSheath previously designed for the SAPIEN 3.
There is very little doubt that in the near future the SAPIEN 3 Ultra will be worthy of its name with a valve crimped on the balloon using a dedicated delivery system and an eSheath living up to our expectations.
Conflict of interest statement
T. Lefèvre reports being a proctor for Edwards Lifesciences, Boston Scientific and Abbott.
Supplementary data
To read the full content of this article, please download the PDF.

