An 87-year-old woman with severe symptomatic aortic stenosis was selected for TAVI by the Heart Team because of a high surgical risk (log. EuroSCORE: 24.2%). The mean transvalvular gradient was 56 mmHg and the diameter of the aortic annulus 21 mm. Therefore a 26 mm CoreValve™ prosthesis (Medtronic, Minneapolis, MN, USA) was selected. We decided to perform the procedure without prior balloon valvuloplasty. After femoral access with an 18 Fr sheath, a stiff guidewire was placed in the left ventricle. The prosthesis was advanced over the stenotic valve without problems (Figure 1A). The sheath cover was then retracted under fluoroscopic guidance and the valve released. It became apparent that the valve was highly compressed in its distal part and the position seemed optimal, with the distal end of the prosthesis reaching one cell (8 mm) into the left ventricular outflow tract (Figure 1B). We decided to perform a balloon dilatation with a 22 mm Nucleus™ balloon (NuMED, Hopkinton, NY, USA). In the meantime, before the balloon was advanced over the valve, we saw that the valve had moved upwards, reaching now only half a cell below the annulus (Figure 1C). We rapidly performed the balloon valvuloplasty (Online Figure 1) which led to an appropriate expansion of the distal part of the valve, with the distal part of the prosthesis now ending only one to two millimetres below the annulus (Online Figure 2). Angiography showed that there was no residual aortic insufficiency (Online Figure 3).
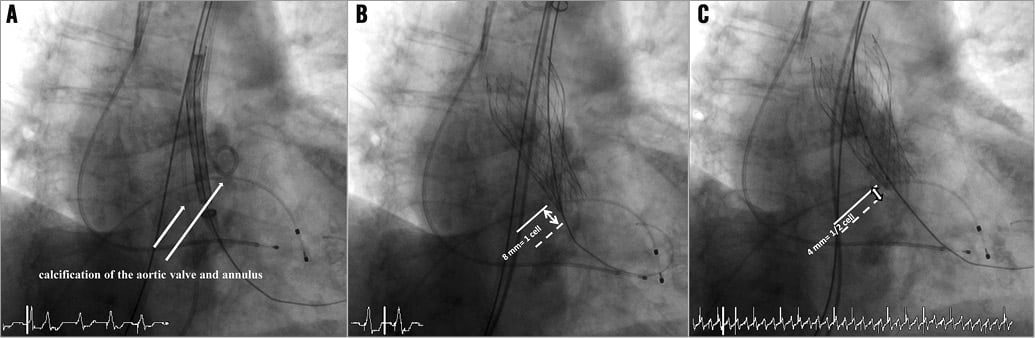
Figure 1. A) Positioning of the Medtronic CoreValve™ prosthesis. B) Result after release of the prosthesis: good placement but compression of the distal part of the prosthesis. C) Result after positioning of a balloon: spontaneous cranial dislodgement of the prosthesis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
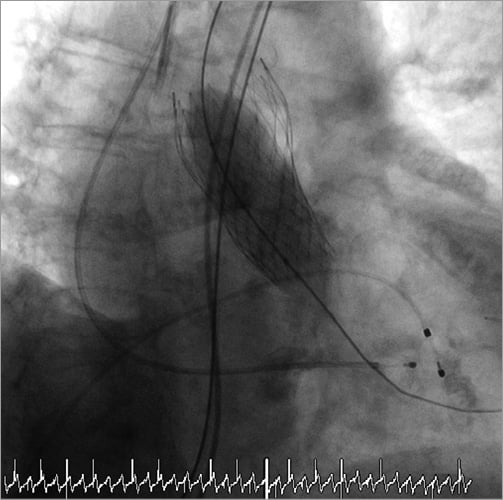
Online Figure 1. Balloon valvuloplasty with a 22 mm Nucleus™ balloon.
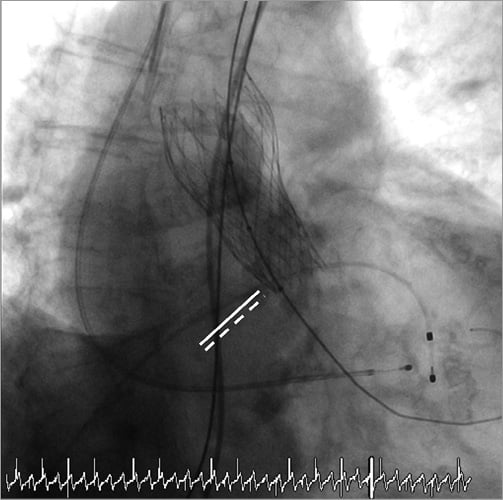
Online Figure 2. Result after balloon valvuloplasty.
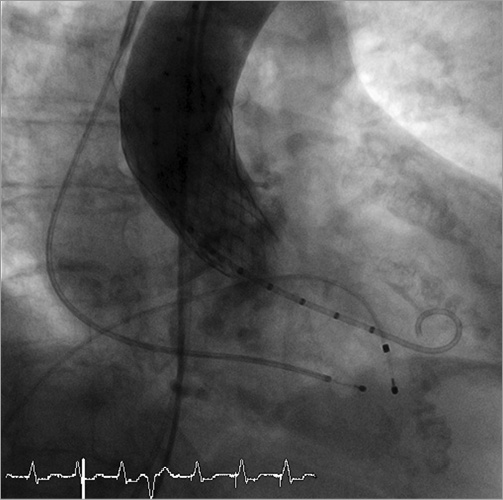
Online Figure 3. Final angiography, demonstrating no residual insufficiency.

