- aortic stenosis
- percutaneous aortic balloon valvuloplasty
- aortic valve replacement
- transcatheter aortic valve implantation
Abstract
Aims: To assess the results of percutaneous aortic balloon valvuloplasty (PABV) as a potential bridge to further intervention in patients referred for transcatheter aortic valve implantation (TAVI).
Methods and results: Two hundred and fifty-three patients referred for TAVI were studied: 41 (16%) were considered transiently unsuitable for either aortic valve replacement (AVR) or TAVI and underwent PABV as a bridge to intervention. In the others, primary TAVI or AVR was performed in 140 cases, and medical therapy alone in 72.The overall population was at high risk: 82±8 years, logistic EuroSCORE: 28±16%, STS score: 16±10%. There was no PABV-related death. Twenty-three patients underwent secondary TAVI (n=19) or AVR (n=4), 18 did not undergo further intervention. One and two year survival rates were respectively 94±5% and 85±10% after bridge PABV, and 33±11 and 6±5% after PABV alone. There was no difference in survival between the primary TAVI / AVR and bridge PABV (p=0.08), and between medical treatment and PABV alone (p=0.36).
Conclusion: In high-risk patients with aortic stenosis and temporary contraindications to AVR or TAVI, PABV may be used as a bridge to intervention with good mid-term outcomes. In others, PABV can be safely used but is associated with a poor outcome.
Abbreviations
AR aortic regurgitation
AS aortic stenosis
AVA aortic valve area
AVR aortic valve replacement LVEF left ventricular ejection fraction
MI myocardial infarction
NYHA New York Heart Association
PABV percutaneous aortic balloon valvuloplasty
PCI percutaneous coronary intervention
SD standard deviation
SPAP systolic pulmonary artery pressure
STS-PROM Society of Thoracic Surgeons Predicted Risk of Mortality
TA transapical
TAVI transcatheter aortic valve implantation
TF transfemoral
Introduction
Since its introduction for treating aortic stenosis (AS) more than 20 years ago, percutaneous aortic balloon valvuloplasty (PABV) has had limited indications because of its poor efficacy in improving the natural history of the disease.1-3 However, this technique has experienced a revival with the development of transcatheter aortic valve implantation (TAVI), as part of the procedure.4,5 Furthermore, it may represent an interesting option as a bridge to TAVI or surgical aortic valve replacement (AVR).6
The aim of this study was to describe the current use and results of PABV in high-risk patients referred for the management of severe and symptomatic AS by TAVI.
Methods
From October 2006 to September 2009, all patients consecutively referred for TAVI underwent a screening process and multidisciplinary clinical evaluation, transthoracic and, if necessary, transesophageal echocardiography, coronary angiography, aortic and femoroiliac angiography, and multislice computed tomography. The decision to perform TAVI was taken in patients with severe symptomatic AS; contraindications to, or high risk for AVR (EuroSCORE ≥20% or STS-PROM ≥10%); a life expectancy >1 year; an anatomy suitable for intervention; no need for coronary bypass surgery.7 In patients with contraindications to TAVI, AVR was reconsidered if the operative risk was not deemed prohibitive. In patients who were too frail, or those with technical contraindications to any invasive intervention, or with comorbidities that limited short-term life expectancy or precluded future quality of life, a simple medical treatment was decided upon. Finally, PABV was considered as a potential bridge to further intervention in patients with either transient or doubtful contraindications to both TAVI and AVR, for example in those whose clinical condition did not allow screening upon admission because of haemodynamic instability, such as cardiogenic shock (arterial hypotension below 90 mmHg, unresponsive to inotropic vasopressors agents, associated with clinical and biological signs of peripheral hypoperfusion or progressive multi-organ failure) or severe pulmonary oedema requiring invasive or non-invasive ventilation. Among the latter patients, some of them actually underwent secondary TAVI or AVR (bridge PABV group), and others had no further intervention (PABV alone group).
Procedures were usually performed under local anaesthesia, with fluoroscopic guidance, via the transfemoral retrograde approach. However, in emergent situations such as cardiogenic shock, 12 patients were treated after oro-tracheal intubation and under general anaesthesia. After placement of a 10 or 12 Fr sheath, the native valve was crossed and a 0.035” extra-stiff 2.6 m length J curved wire was placed at the apex of the left ventricle. Then, a valvuloplasty balloon (Cristal; Balt, Montmorency, France or Nucleus; Numed, Hopkinton, NY, USA) was brought to the aortic valve. The size of the balloon diameter was chosen according to the measurement of the annulus diameter by echocardiography (ratio 1/1). In most cases, a rapid right ventricular pacing was used to stabilise the balloon during inflation. At the end of the procedure, patients were transferred to the intensive care unit.
Hospital clinical and echocardiographic data were prospectively obtained before discharge. All adverse events were recorded. After the hospital phase, clinical follow-up was obtained in all survivors at 1-3 months, 6 months, 1 year, and then annually.
Continuous data were expressed as mean±SD, except for the delay to TAVI or AVR after PABV, and for the length of follow-up, which were expressed as median with 25th-75th percentiles. The Mann-Whitney U test was used to compare continuous variables in the different groups, and categorical variables were compared by the chi-square or Fisher exact test. Multiple group comparisons used one-way analysis of variance or chi-square test and, when significant, 2-by-2 subgroup comparisons were performed using the Bonferroni correction. Survival rates were estimated using the Kaplan-Meier method and were compared using the log-rank test. All tests were two-sided. A p-value <0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using statistical software Statistica version 5.0; Statsoft Inc., Tulsa, OK, USA.
Results
The management of the study population is detailed in Figure 1. Of the 253 patients at high risk or with contraindications to surgery who were referred for TAVI, 41 (16%) underwent PABV because of: unstable haemodynamic state requiring urgent intervention and precluding screening process in 27 cases (of whom 12 were rescue PABV for cardiogenic shock); restricted availability of the device in six cases; associated cancer requiring further explorations in four cases; therapeutic test for contentious clinical presentations in three cases (combination with severe lung disease in two cases; uncertainty over the degree of AS in one); associated unstable coronary artery disease requiring urgent percutaneous revascularisation combined with PABV in one case. Among the other patients referred for TAVI, 140 patients underwent primary TAVI (n=113, 45%) or AVR (n=27, 10%), 62 (25%) received no intervention because of general or technical contraindications to any intervention, and 10 (4%) died before intervention.
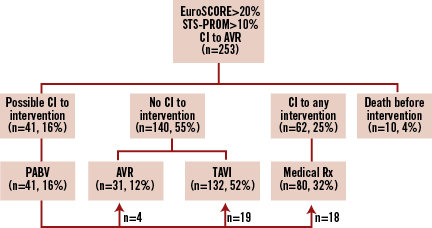
Figure 1. Management of high-risk patients with severe symptomatic aortic stenosis. Flow chart of 253 high-risk patients referred for transcatheter aortic valve implantation. AVR: aortic valve replacement; CI: contraindication; PABV: percutaneous aortic balloon valvuloplasty; TA: transapical; TAVI: transcatheter aortic valve implantation TF: transfemoral
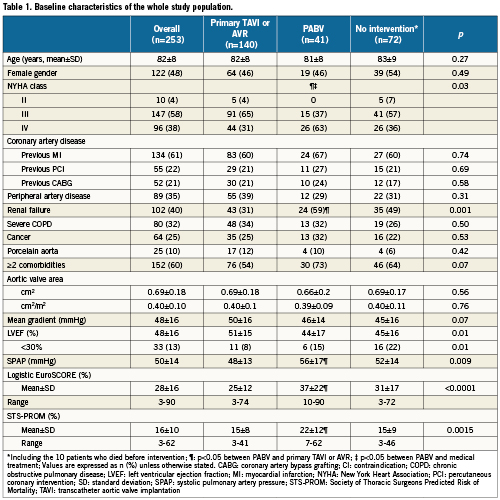
The characteristics of the whole population are presented in Table 1. Overall, patients treated by PABV had a higher risk profile than those who were treated using primary TAVI or AVR, regarding the severity of NYHA class, the presence of renal failure and other extra-cardiac comorbidities, which was reflected by higher logistic EuroSCORE and the STS scores. Conversely, the echocardiographic parameters addressing the severity of the AS and left ventricular function did not significantly differ between the groups. The characteristics of the patients treated by bridge PABV and PABV alone are presented in Table 2. There was no difference between the two groups except for renal failure, which was more frequent in patients treated by PABV alone than by bridge PABV, and the trend towards higher risk scores in the PABV alone group.
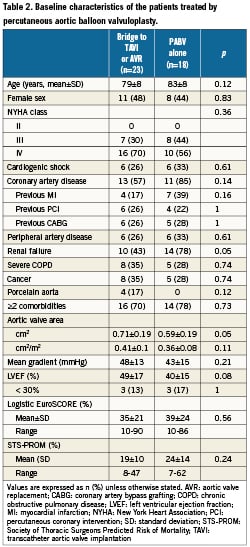
Of the 41 patients who underwent PABV, 23 underwent secondary elective TAVI (n=19: TF, n=15, TA, n=4) or AVR (n=4, bioprostheses), while 18 finally did not undergo further intervention because of technical contraindications to both transfemoral and transapical TAVI in 10 cases (too large aortic annular diameters: n=9, severe respiratory failure: n=1), general contraindications secondarily detected by further geriatric evaluation in five (disseminated cancer: n=3, frailty: n=1, severe cognitive disorder: n=1), in-hospital death before intervention in two cases and patient refusal in one case. The median delay between bridge PABV and TAVI or AVR was 48 days (4-202): it was <10 days in three patients (after rescue PABV), 10 to 30 days in six, one to six months in eight, and >6 months in six. It was significantly shorter in patients with initial cardiogenic shock than in stable patients (median 12 days [8-28] versus 145 days [35-442], respectively, p= 0.002).
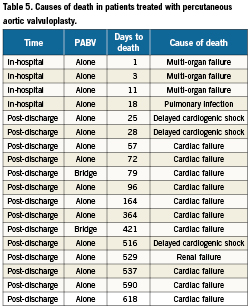
Early outcomes are detailed in Table 3. PABV was successfully performed in all the cases and complications were rare. The most frequent complication was the occurrence of new complete atrioventricular blocks requiring pace-maker implantation (7%). There was one vascular complication (2%) in an 87-year-old woman who presented a voluminous haematoma at the femoral puncture site requiring surgical drainage. There was neither stroke nor myocardial infarction. No death occurred during PABV. Haemodynamic results of PABV are presented in Table 4. All parameters were significantly improved by the procedure and, overall, there was no significant change in the AR grade. There was only one grade 3 AR, with no early clinical consequences. When it was initially <40%, LVEF increased in all the cases after PABV (Figure 2). Thirty-day mortality was 15% (6/41) consisting of four in-hospital deaths and two post-discharge deaths at day 25 and day 28 from delayed cardiogenic shock; all the deaths were observed in the PABV alone group. All the in-hospital deaths were observed in the 12 patients with initial cardiogenic shock: three patients were not improved by PABV and died promptly from multi-organ failure while one died at day 18 from pulmonary infection. The eight other patients with cardiogenic shock treated by rescue PABV improved and were subsequently treated by TAVI in four cases and by AVR in two cases, while two others did not undergo further intervention. As a result, 30-day mortality was 33% (4/12) for patients treated by rescue PABV for cardiogenic shock. Of the 37 patients who were discharged alive from hospital, clinical improvement (decrease in ≥1 NYHA class) was present in 33 patients (89%); three patients (8%) remained in class III, one (3%) in class IV. Thirty-day mortality was 12% (17/140) in patients treated by primary TAVI or AVR, and 14% (10/72) in those receiving a simple medical treatment.
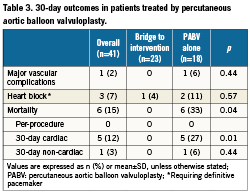
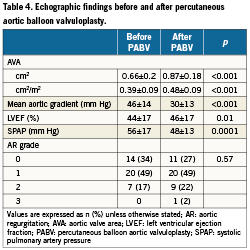
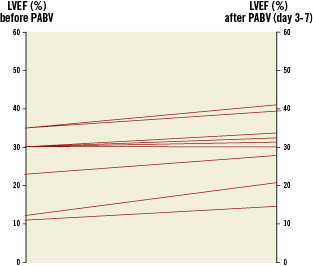
Figure 2. Percutaneous aortic balloon valvuloplasty and ventricular function. Impact of percutaneous aortic balloon valvuloplasty on depressed left ventricular ejection fraction (<40%). LVEF: left ventricular ejection fraction; PABV: percutaneous aortic balloon valvuloplasty
Median follow-up duration was 10 months (2-18) in patients who underwent primary PABV. The Kaplan Meier survival curves of all patients’ subsets are presented in Figure 3. One and 2-year survival rates were respectively 94±5 % and 85±10 % after bridge PABV, and 33±11 % and 6±5 % after PABV alone. The causes of deaths are detailed in Table 5. All but two of them were observed in the PABV alone group and they were mostly due to delayed progressive cardiac failure. Of the 27 patients who presented initially with haemodynamic instability and finally underwent TAVI or AVR, one died at day 421 (heart failure). There was no late death in patients who initially presented with cardiogenic shock. Two-year survival rates were 65±7% after primary TAVI or conventional AVR, and 17±7% after medical treatment alone. Overall, there was no significant difference in survival rates between the primary TAVI/AVR and bridge PABV groups on one hand (p=0.08), and the medical treatment and PABV alone groups on the other (p=0.36).
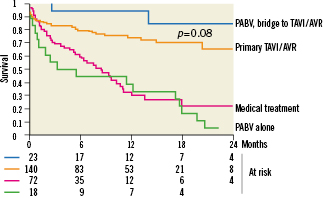
Figure 3. Two-year follow-up survival curves. Kaplan Meier survival curves in 253 high-risk patients with aortic stenosis, according to their treatment. AVR: aortic valve replacement; PABV: percutaneous balloon aortic valvuloplasty; TAVI: transcatheter aortic valve implantation
Discussion
Very few data exist on the use of PABV as a bridge therapy to TAVI or AVR.8-10 In patients with temporary or doubtful contraindications to both TAVI and AVR, PABV was used as a potential bridge to these interventions. Despite more severe baseline risk profiles, bridge PABV led to short- and mid-term results similar to those observed in patients treated by primary TAVI or AVR. However, when patients could not receive secondary interventions, PABV did not improve the prognosis when compared to medical therapy.
With the emergence of TAVI, PABV has had to be performed as part of the procedure, which has led to refinement of the materials used and to greater operators’ skills11,12 In a series on 141 patients, Agatiello et al reported 4% in-hospital mortality, and a 6% non-fatal complications rate.13 These conditions may lead to a reconsideration of PABV to achieve clinical improvement while awaiting the availability of subsequent treatment. Our results confirm that, despite the high-risk profile of these patients, the current use of PABV is reasonably safe. The most frequent event consisted in a 7% rate of new complete atrioventricular blocks requiring a pacemaker, which should be taken into account while interpreting conduction disturbances observed after TAVI.
Contrary to the improvement of procedural safety, haemodynamic efficacy remained limited in the most recent series, with an increase of approximately 40 to 50% in effective orifice area and decrease in mean transvalvular gradient, which is consistent with the present results. However, these modest valve changes were sufficient to achieve significant improvements in LVEF, pulmonary pressures and clinical condition.
Nearly 20 years ago, Cribier et al and Moreno et al pointed out the potential lifesaving role of PABV as an initial treatment for patients with cardiogenic shock due to critical AS.14,15 However, patients were younger than in the present series, with a less severe risk profile. The study by Smedira et al also suggested favourable outcomes with this strategy in a series of five patients, and Zimrin et al reported one case of PABV as a bridge to aortic valve bypass with an apicoaortic conduit.16,17 More recently, Ussia et al showed that bridging TAVI with PABV was feasible and reasonably safe to offer temporary relief in selected patients with a high chance of periprocedural complications, and Hamid et al tended to the same conclusions.8,9 Doguet et al showed that, used as a bridge to AVR, PABV improved cardiac function and the postoperative course.10 Many patients at high risk are now referred for severe AS18 but TAVI may not be immediately possible due to various reasons: 1)uncertainty as to the degree of aortic stenosis or comorbidities which could preclude reasonable life expectancy. PABV may be an interesting option to allow completion of the work-up before discussing the definitive treatment; 2) very low LVEF or severe pulmonary hypertension, contributing to an increase in the risk of TAVI, as recently shown by Rodés-Cabau et al19 PABV may improve LVEF and decrease the pulmonary pressures; 3) instability of haemodynamic state, cardiogenic shock or acute coronary syndrome requiring emergent or urgent intervention. Due to availability of the device and logistic considerations, emergent TAVI is not practically doable in most centres, and it has not been evaluated so far. In this situation, TAVI may have been used in combination with left ventricular assistance device; however, need for haemodynamic support has also been identified as a predictor of mortality after TAVI;19,20 4) In the early phase of the program, access to the device may be limited. This accounted for the fact that six patients had to wait more than six months between PABV and TAVI. The patients treated by bridge PABV were at very high risk in this series: 70% of them were in NYHA class IV, 23% were in cardiogenic shock and the average predicted mortality was 35% according to the EuroSCORE, and 19% according to the STS-PROM. This risk profile was much more severe than that of patients who underwent primary TAVI or AVR. Although most patients finally underwent TAVI, clinical improvement achieved by PABV led to opting for conventional AVR in some cases, which was also observed by Kapadia et al.6 Despite the more severe risk profile, the outcomes of TAVI or AVR after bridge PABV were similar to primary TAVI or AVR. The significant decrease in pulmonary hypertension and increase in LVEF observed after PABV may have contributed to this result. Also, the fact that patients with decompensated heart failure with no shock and those with severely depressed LVEF were treated by primary TAVI/AVR may explain this finding.
Previously reported results of PABV concluded that results were poor because of the conjunction of a high procedural complications rate, modesty of haemodynamic improvement, and valve restenosis. The study by Bernard et al showed the poor long-term prognosis of patients treated by PABV alone as compared to those treated by AVR (47% versus 83% 2-year survival rates).21 As PABV could not change the natural history of AS, its indications were drastically limited as the sole treatment in selected cases to alleviate refractory symptoms.1-3,22-24 In the present study, PABV was never proposed in patients who had been upfront denied any definitive intervention. However, some patients who were initially treated by PABV with the intention of carrying out further interventions, could not receive them. There was no significant difference in baseline characteristics between patients treated by PABV alone and those treated by bridge PABV. This suggests that differences between both groups mainly related to qualitative, subtle parameters, such as frailty or nature of comorbidities, which could not be immediately detected upon admission and could hardly be quantified and reflected by the predictive risk scores. Overall, the outcomes of patients treated by either PABV alone or medical therapy were similar, and, one and two-year mortality rates were dismal in both groups. The present 33% one-year survival rate observed in patients treated by PABV alone is consistent with those reported by Svensson et al and by Otten et al in US and European experiences in similar populations.25,26 This confirms previous findings demonstrating the inability of PABV alone to improve the long-term prognosis of severe symptomatic AS.
Because of its observational nature, the present study does not allow the establishment of a definite reference treatment strategy as regards the use and timing of bridge PABV, which would require a randomised comparison with primary TAVI. This study reflects a single-centre experience of a relatively limited number of patients. However, this allowed a uniform management of the whole study population, as well as comprehensive and prospective data collection with no patient lost to follow-up. As indications for PABV were selected on a case-by-case basis, the study can neither lead to definitely conclude about the usefulness of this strategy, nor to clearly identify the population that might benefit from PABV before TAVI or AVR.
Conclusion
Today, the safety of PABV is good, with improved technical evolutions and operators’ experience due to the current diffusion of TAVI. Bridge PABV safely allows for a waiting period before secondary intervention when further explorations are needed, and is clinically effective when haemodynamic instability requires emergent intervention. This strategy achieves good short- and mid-term outcomes, with regard to the severity of the risk profile of patients. As a sole treatment, PABV is unable to interfere with the poor long-term prognosis of the disease. In the future, efforts should be made to better stratify patients’ prognosis upon admission and determine those who should undergo bridge PABV and those who could receive primary TAVI.
Conflict of interest statement
D. Himbert and P. Nataf are proctors for Edwards Lifesciences. A.Vahanian and B. Iung received speaker’s fees from Edwards Lifesciences. The other authors have no conflict of interest to declare.

