The intra-aortic balloon pump (IABP) has been the most used mechanical circulatory support (MCS) device in the last 40 years. Its utility has been investigated in several clinical settings: cardiogenic shock, myocardial infarction, high-risk percutaneous coronary interventions, left ventricular unloading, and as a bridging strategy to high-risk cardiac surgery. However, no data on the feasibility of temporary MCS with an IABP during transfemoral transcatheter aortic valve implantation (TAVI) are available.
From November 2021 to November 2022, eleven patients with severe aortic stenosis (AS) and a left ventricular ejection fraction (LVEF) <30% (low-flow, low-gradient: n=7) underwent elective transfemoral TAVI with prophylactic transfemoral IABP support. Patients had a median age of 82 years (interquartile range [IQR]: 71.8-85.3), two of the eleven (18%) were female, the median LVEF was 20% (IQR: 18%-25%), and four patients (36%) had known coronary artery disease. Ten balloon-expandable valves (SAPIEN 3 Ultra; Edwards Lifesciences) and one self-expanding valve (Evolut PRO+; Medtronic) were implanted. No patients underwent balloon predilation. Procedural success with no haemodynamic instability or perioperative complications (i.e., cerebrovascular or access-site related complications) was achieved in all patients; the IABP was removed on the same day (n=7) or on day 1 post-procedure (n=4).
Prophylactic IABP counterpulsation was initiated during the index procedure preceding conventional TAVI. The IABP was inserted through the contralateral femoral artery access to the artery reserved for subsequent transcatheter heart valve (THV) delivery. Radial artery access was also obtained for pigtail catheter placement. After confirmation of accurate IABP positioning in the descending aorta under fluoroscopic imaging, standard TAVI protocol was followed. Because of the highly compliant properties of the IABP, neither the THV tracking in the descending aorta nor the proper functioning (i.e., balloon inflation and haemodynamic support) of the IABP were compromised (Figure 1, Moving image 1). Immediate IABP use was deferred in patients with greater than mild aortic insufficiency because of the risk of worsening valve insufficiency and acute left ventricular volume overload. In this subset of patients, IABP MCS was initiated after THV deployment. Otherwise, the IABP was kept functioning throughout the procedure, with the sole exception being during the balloon-expandable valve deployment under rapid pacing (Central illustration, Moving image 1).
TAVI is a consolidated treatment providing survival benefit to patients with severe AS. Yet, those with a severely reduced LVEF, especially in the case of low-flow, low-gradient AS, represent a group with increased mortality1. IABP-induced diastolic pressure augmentation might be particularly beneficial in patients with left ventricular dysfunction, low-flow state, and coronary artery disease. This pilot experience demonstrates the feasibility of temporary MCS with an IABP during transfemoral TAVI, with no impact on valve delivery through the descending aorta2. Although these findings are promising, prospective studies are warranted to validate the safety and efficacy of TAVI with prophylactic IABP support.
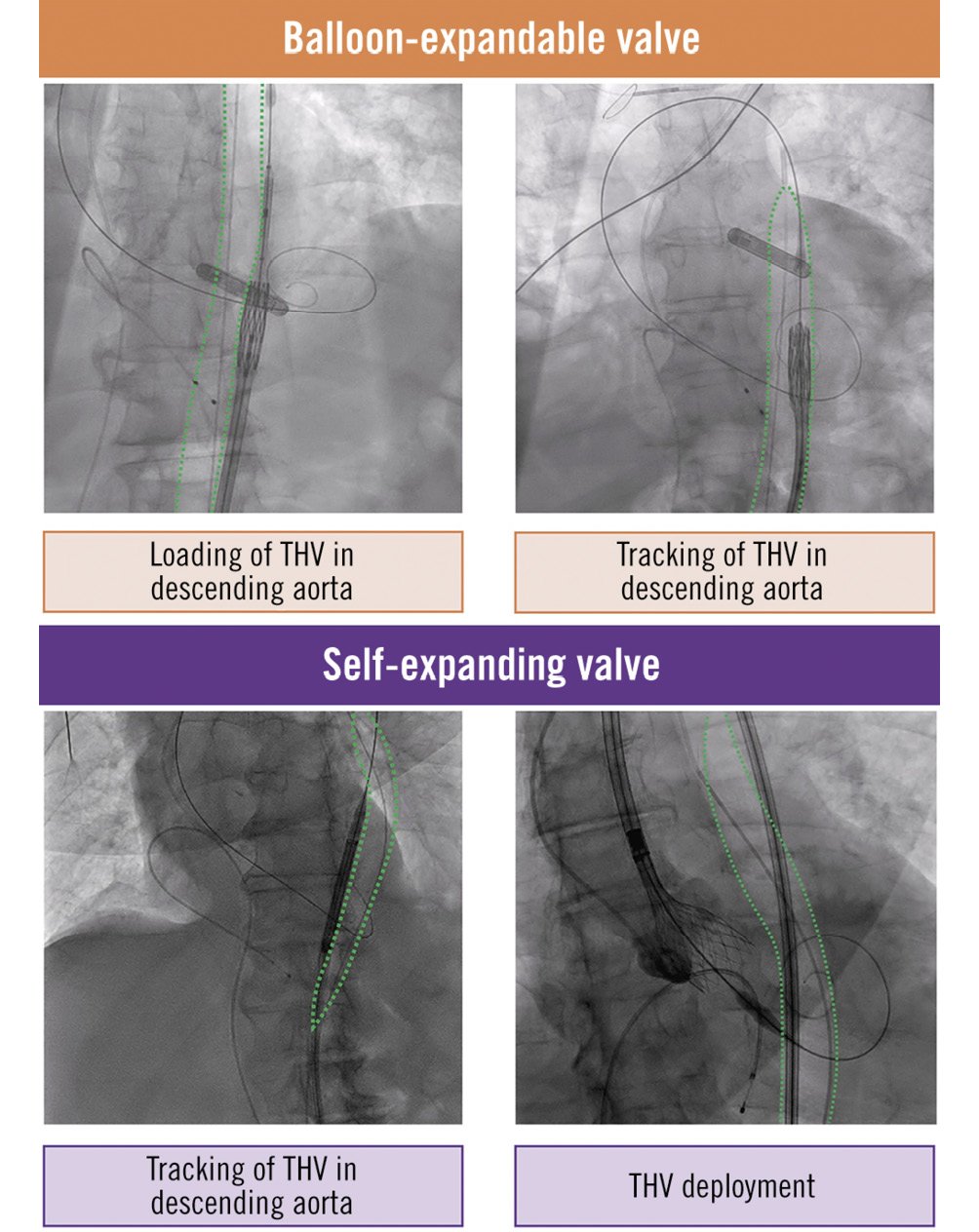
Figure 1. The intra-aortic balloon pump can be kept functioning during the following procedural steps: loading of transcatheter heart valve (THV) in descending aorta (balloon-expandable THV), tracking of THV in descending aorta (balloon-expandable and self-expanding THV), and THV delivery (self-expanding THV).
Conflict of interest statement
A. Latib has served on advisory boards or as a consultant for Medtronic, Boston Scientific, Philips, Edwards Lifesciences, and Abbott. S. Ludwig has received travel compensation from Edwards Lifesciences and consulting fees from Bayer. The other authors have no conflicts of interest to declare related to the content of this paper.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Transcatheter aortic valve implantation with a prophylactic intra-aortic balloon pump.

