
Introduction
The transaxillary (TAx) arterial access is used for transcatheter aortic valve implantation (TAVI) when the transfemoral (TF) access is inappropriate.
The TAx access is traditionally obtained through surgical cut-down, with the patient under general anaesthesia. More recently, a percutaneous TAx procedure has been developed1. At our centre, a modified percutaneous TAx approach has been used as an alternative access since 2017 using the novel MANTA® (Essential Medical [now Teleflex], Malvern, PA, USA) vascular closure device (VCD) to achieve haemostasis2. The MANTA is approved for vascular closure after TF TAVI but so far there is limited experience with the MANTA VCD in percutaneous transaxillary access.
Methods
The aim of this study was to assess the safety and efficacy of percutaneous transaxillary artery access TAVI using the MANTA VCD to achieve haemostasis.
This single-centre, retrospective study examined all 16 cases of percutaneous TAx access TAVI at our centre between June 2017 and November 2018. No eligible patients were excluded from the analysis.
Percutaneous TAVI was preferably performed via the left axillary artery. The technique is similar to the Hamburg technique1 and has been described previously2. A 0.035” 260 cm hydrophilic wire (GLIDEWIRE®; Terumo Corp., Tokyo, Japan) was placed from an ipsilateral 6 Fr radial artery sheath and snared out (Amplatz Goose Neck™; Medtronic, Minneapolis, MN, USA) through an 8 Fr femoral artery sheath. Ultrasound-guided micro-puncture of the axillary artery was followed by 6 Fr arterial sheath insertion. An Amplatz Ultra-Stiff 0.035” wire (Cook Medical, Bloomington, IN, USA) was placed in the ventricle and the 18-20 Fr arterial sheath was introduced to the aortic arch. An Evolut™ R or Evolut™ PRO self-expanding valve prosthesis (Medtronic) was delivered after predilatation. A 6-7 mm balloon was inserted over the hydrophilic wire from the radial sheath to a position in the axillary artery distal to the puncture point. Thereafter, the delivery system and the large-bore sheath were removed and an 18 Fr MANTA VCD was deployed to the arteriotomy site in the same fashion as in TF access. The success of the haemostasis and absence of stenosis was checked immediately with a contrast injection (Figure 1). If there was overt bleeding, the 6-7 mm balloon was inflated to obtain temporary haemostasis, followed by a covered stent (VIABAHN®; Gore Medical, Newark, DE, USA) over the arteriotomy site, using the wire from the femoral access. Unfractionated heparin was administered to maintain an activated clotting time of 250-300 seconds, which could be reversed with protamine in case of bleeding.
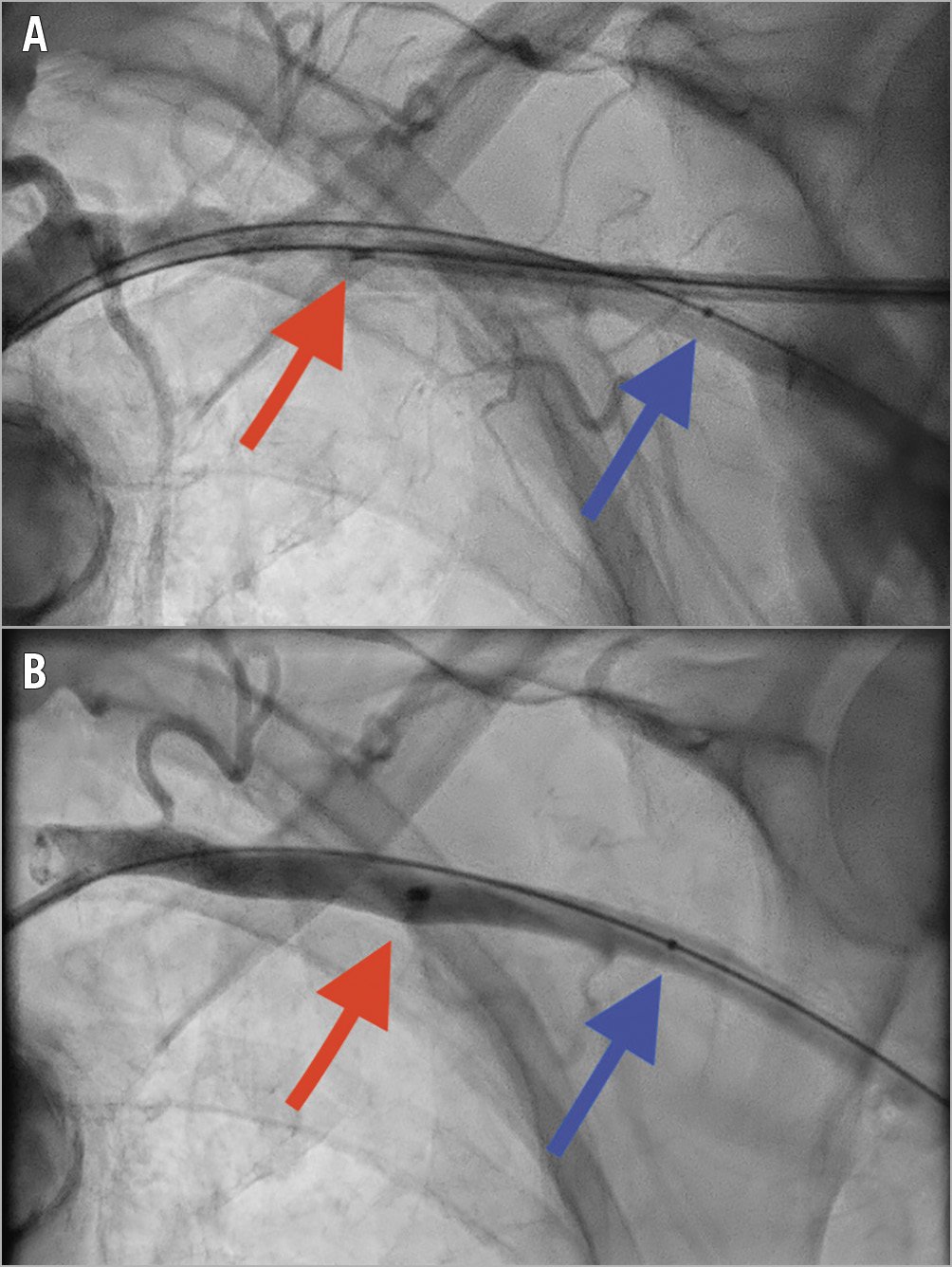
Figure 1. Angiographic images of the left axillary artery. A) Deployment of the MANTA vascular closure device (red arrow). From the radial access a 7 mm balloon (blue arrow) is positioned over the hydrophilic wire. B) Good haemostasis. The red arrow shows the metal marker on the MANTA device.
Data were collected from the Swedish TAVI registry, hospital records, radiological examinations from the procedures and preprocedural examinations of the access arteries. Outcomes were analysed according to VARC-2-defined endpoints if not stated otherwise3.
The study was approved by the Ethics Board in Stockholm and performed in accordance with the Declaration of Helsinki and did not require the patients’ written consent.
Results
The study population was typical for TAVI (average age 83 years, Society of Thoracic Surgeons risk score for mortality 7%); 75% were female. The minimal axillary artery diameter on the used side was 5.6 mm (less than 5.5 mm in eight patients). Left-sided axillary access was used in 15 out of 16 patients, and general anaesthesia was used in the first 12 patients, with the last four treated under local anaesthesia only. Fourteen patients were treated with the Evolut R system and two with the Evolut PRO system. All valves were delivered through sheaths, the most common being 18 Fr. All cases used predilatation of the aortic valve. Average fluoroscopy time was 33 minutes and contrast volume 85 ml.
The MANTA VCD was used in 15 out of 16 patients. In one patient, the operator used a covered stent as the primary haemostasis method without a prior attempt at reaching haemostasis with the MANTA. There was a minor vascular complication consisting of closure device failure in 47%. In the first eight patients, the MANTA failed in three, and in the last seven patients the MANTA failed in four. In all cases, a covered stent was deployed successfully, and there was no need for vascular surgery. There was no stenosis in the final angiogram of the axillary artery access site.
There was one case of a major vascular complication in combination with major bleeding, a dissection of the axillary artery distal to the vascular closure device, which was solved by stenting.
There were two other major bleedings not associated with the transaxillary access (haematuria after a urinary catheter, and bleeding after a central venous catheter).
One patient suffered a large postoperative stroke which was ultimately fatal. There was no other 30-day mortality.
Discussion
We studied the first 16 cases of transaxillary TAVI with percutaneous access at our institution. Almost half of the patients did not achieve immediate haemostasis with the MANTA VCD. Although placement of a covered stent solved the problem and only one case of major bleeding was associated with the access site, this is a much higher failure rate than in transfemoral TAVI4.
Reasons for this higher failure rate in TAx TAVI might be that manual compression of the access site is more difficult in the transaxillary access. It is also possible that a routine balloon occlusion of the access site for 5-10 minutes after MANTA deployment would have reduced the number of failures. In half of the patients, the axillary artery diameter was less than 5.5 mm, which is lower than the recommended artery size for the MANTA, and might have contributed to a higher failure rate.
In the largest published experience with a VCD after TAx TAVI, another closure device (ProGlide®; Abbott, Abbott Park, IL, USA) had a lower failure rate1.
Since two of the three major bleeding complications were not related to the transaxillary access site but to urinary and central venous catheters, performing the procedure under local anaesthesia in a more minimalist way might decrease bleedings.
Limitations
This was a small single-centre experience which might have limited the generalisability.
Conclusion
Percutaneous transaxillary TAVI using the MANTA VCD was associated with a high failure rate, although placement of a covered stent achieved haemostasis in all cases.
|
Impact on daily practice This study shows that percutaneous access and closure in transaxillary TAVI is safe. However, the MANTA vascular closure device seems to have limitations in transaxillary access. |
Conflict of interest statement
A. Rück has received lecture honoraria, research support and proctor fees from Boston Scientific and Edwards Lifesciences. M. Settergren has received lecture honoraria and proctor fees from Abbott, Boston Scientific, Medtronic, W.L. Gore and Edwards Lifesciences. N. Saleh and R. Linder have received lecture honoraria and proctor fees from Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

