Vascular closure devices (VCD) have been compared to manual compression for 20 years in the setting of both diagnostic and interventional cardiovascular procedures1. The endpoints of comparison have been threefold: 1) time to haemostasis, 2) time to ambulation, and 3) rate of complications. The approval of closure devices has required proof of efficacy, not safety. Thus, all approved closure devices have demonstrated a decrease in either time to haemostasis, time to ambulation or both of these efficacy endpoints compared to manual compression. The history of VCD approval and utilisation has been marred by an inherent controversy: as the initial trials were not required to demonstrate either non-inferiority or superiority of these intravascular and extravascular femoral devices with respect to any safety endpoint, it is reasonable for clinicians, agencies and societies to ask a simple question –are the millions of implanted vascular closure devices safe?
Smaller (N=4,000) meta-analyses of clinical trials have questioned the safety of VCD and have led to warning statements by societies such as the American Heart Association2,3. More recent meta-analyses incorporating over 7,000 patients in randomised trials continue to express some caution regarding device safety4. On the other hand, the Food and Drug Agency in the United States has asked this question repeatedly by pooling clinical trials and registries. The answer reaffirmed the safety of VCD with the exception of a caution regarding the VasoSeal extravascular plug device (Datascope Corp., Montvale, NJ, USA)5,6. These varying results are due to mixed populations, mixed definitions, mixed inclusion criteria (trials, registries or both) and ultimately a meta-analysis is unlikely ever to settle the question of VCD safety.
Back to the beginning: vascular closure device trials
When so much doubt exists regarding the safety of an interventional cardiology device, meta-analyses and registry studies are unlikely to resolve the controversy. The need to prove the safety of VCD is more significant now than ever before. While a VCD is a potentially significant bleeding avoidance strategy, there is competition from another 20-year-old approach (radial artery access) in which large randomised clinical trials have been more clearly powered to look at safety7. Furthermore, the efficacy endpoints of VCD are easy to prove but clinically questionable. Does it matter to the patient if time to haemostasis is one minute versus 10 minutes? At hospitals which fix bed rest to two to four hour periods, the advantage of a one-hour VCD ambulation potential in terms of patient satisfaction and hospital efficiency is not necessarily clear.
As we look at the history and approval process of VCD, we see numerous well conducted clinical trials with small sample sizes (N=100-500) for which only efficacy variables could be statistically analysed with any degree of certainty (Table 1)8-10. The interventional cardiology community wants more: are VCD safer than manual compression, yes or no? There are numerous stated hindrances to running adequately sized clinical trials to answer this question that have surfaced over the past 20 years, including economic concerns by industry related to the relatively low profit margin and price of closure devices, difficulty in enrolment, and lack of a clear definition of relevant bleeding complications11.
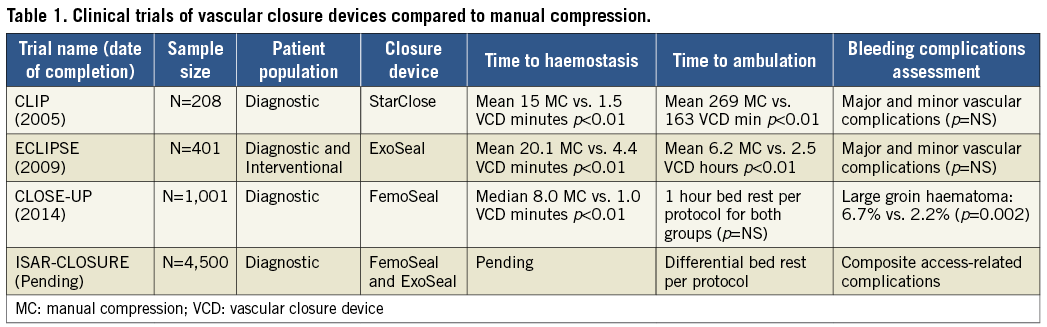
This reticence has hopefully been put to rest by the CLOSE-UP trial group (Closure Devices Used in Everyday Practice)10. In only 13 months, they enrolled 1,014 patients and randomised them to an intravascular closure device, FemoSeal™ (St. Jude Medical, St. Paul, MN, USA), as compared to standardised manual compression. Note that these authors threw out the comparison of time to ambulation by mandating bed rest for only one hour in both groups of patients undergoing diagnostic catheterisation. The typical eightfold reduction in time to haemostasis is seen with FemoSeal in this trial but again the clinical relevance of this finding (eight minutes vs. one minute) is somewhat unimpressive. On the other hand, CLOSE-UP makes a clear statement regarding safety: FemoSeal is superior to manual compression in preventing large (>5 cm) haematomas with a threefold reduction in a safety endpoint. This finding is very important: the AHA warning regarding safety of VCD is largely related to the 4,000-patient meta-analysis by Koreny et al –in this analysis the risk seen with VCD was entirely related to enhanced incidence of haematomas2,3. Quoting these old meta-analyses is no longer required: for the first time, we have proof that a VCD is effective and safe with respect to the most frequent access-site-related complication: haematoma formation.
Finishing the first chapter on VCD safety
In the current issue of EuroIntervention, Xhepa et al demonstrate a trial design that can finish what might have been the first chapter written on VCD12. Building upon CLOSE-UP, the ISAR-CLOSURE (Instrumental Sealing of Arterial Puncture Site-Closure device versus manual compression) trial provides a randomised clinical trial with more patients (N=4,500) than influential meta-analyses on this topic from the not so distant past. At its simplest, ISAR-CLOSURE is designed to provide confirmation of the basic question of safety. Like the CLOSE-UP group, efficacy parameters of time to ambulation are diminished in importance, bed rest of two hours (closure device) versus six hours (manual compression) by protocol guarantees the outcome of the time to ambulation efficacy comparison. While the primary safety endpoint of ISAR-CLOSURE is not exactly the same as in prior small trials and CLOSE-UP, it is likely to be similarly driven by large haematoma and thus the estimated event rates (5% event rate for manual compression) are realistic.
Randomisation does not guarantee absence of controversy. Given the superiority of CLOSE-UP with respect to safety of VCD, the use of a non-inferiority design as opposed to a superiority design will generate discussion. Furthermore, the focus on a low-risk group (diagnostic) as opposed to a PCI group may hamper the event rate comparison and one could wonder if an event-driven trial with an option for expanded enrolment might be considered. Finally, some may argue that large haematomas simply do not matter. While major bleeding complications have been related to the risk of early and one-year death11, the inclusion of large haematoma as part of a death-predicting bleeding characteristic is controversial13. However, this controversy is overstated: let’s assume a large haematoma does not predict death. The softer endpoints of bleeding (bruising, haematoma, epistaxis) have been shown to impact on quality of life for patients14,15. Thus, finishing the chapter on the safety of VCD with respect to even a soft endpoint of reducing haematomas may be relevant to clinicians and patients.
Why perform the ISAR-CLOSURE trial at all, given the positive results of the CLOSE-UP trial? The answer is not simply to provide confirmation and inform guideline statements regarding VCD safety. The design of ISAR-CLOSURE is unique as it provides two devices for comparison: one is an intravascular device (FemoSeal) already demonstrated as superior to manual compression. The other device (ExoSeal®; Cordis, Johnson & Johnson, Warren, NJ, USA) is a plug-based extravascular device that has been studied in a smaller randomised clinical trial that was not powered to comment on safety9 (Table 1). While the concept of extravascular closure has some inherent appeal, ISAR-CLOSURE will be providing important new information. The FDA analysis demonstrated that only one device conferred increased risk compared to manual compression: the extravascular plug device, VasoSeal6. Whether current extravascular plug devices –Mynx (AccessClosure, Inc., Santa Clara, CA, USA), ExoSeal– have overcome the difficult technical challenge of maintaining physical attachment to the mobile, twisting femoral artery is an open question that needs to be answered in an appropriately sized safety oriented trial. After 20 years of VCD utilisation, the ISAR-CLOSURE trial will finish what could have been our first chapter on these devices: we will confirm or deny the safety profile of these devices and point out similarities and differences with respect to extravascular versus intravascular designs. Based upon this new foundation, we can then move on to high-risk groups (interventional patients, >6 Fr sheaths, women) and new VCD designs knowing that efficacy without proof of safety will never be satisfactory.
Conflict of interest statement
Harold L. Dauerman is a consultant for Medtronic and The Medicines Company and has received research grants from Abbott Vascular and Medtronic.

