Abstract
Background: Whether ultrasound (US)-guided femoral access compared to femoral access without US guidance decreases access site complications in patients receiving a vascular closure device (VCD) is unclear.
Aims: We aimed to compare the safety of VCD in patients undergoing US-guided versus non-US-guided femoral arterial access for coronary procedures.
Methods: We performed a prespecified subgroup analysis of the UNIVERSAL trial, a multicentre randomised controlled trial of 1:1 US-guided femoral access versus non-US-guided femoral access, stratified for planned VCD use, for coronary procedures on a background of fluoroscopic landmarking. The primary endpoint was a composite of major Bleeding Academic Research Consortium 2, 3 or 5 bleeding and vascular complications at 30 days.
Results: Of 621 patients, 328 (52.8%) received a VCD (86% ANGIO-SEAL, 14% ProGlide). In patients who received a VCD, those randomised to US-guided femoral access compared to non-US-guided femoral access experienced a reduction in major bleeding or vascular complications (20/170 [11.8%] vs 37/158 [23.4%], odds ratio [OR] 0.44, 95% confidence interval [CI]: 0.23-0.82). In patients who did not receive a VCD, there was no difference between the US- and non-US-guided femoral access groups, respectively (20/141 [14.2%] vs 13/152 [8.6%], OR 1.76, 95% CI: 0.80-4.03; interaction p=0.004).
Conclusions: In patients receiving a VCD after coronary procedures, US-guided femoral access was associated with fewer bleeding and vascular complications compared to femoral access without US guidance. US guidance for femoral access may be particularly beneficial when VCD are used.
Introduction
Transradial access reduces major vascular complications and access site bleeding compared to transfemoral access in patients undergoing coronary angiography or percutaneous coronary interventions (PCI) and is therefore recommended1. However, femoral access is still indicated in larger bore procedures, in procedures where radial access failure has occurred, and bilateral internal mammary coronary graft angiography. Vascular and bleeding complications in femoral access can occur because of high or low punctures, multiple punctures, or inadequate haemostasis technique2. Studies have demonstrated that vascular closure devices (VCD) are associated with a reduced time to haemostasis and reduced time to ambulation, with no difference in the incidence of vascular injury or mortality compared to manual compression345. Given the ubiquitous use of vascular closure devices for femoral access to optimise patient comfort and increase workflow, strategies to decrease vascular closure device complications are clinically important.
The UNIVERSAL trial, which randomised 621 patients, stratified by planned VCD use, to ultrasound (US) versus no US for femoral access, did not demonstrate a significant difference for the primary composite outcome of major bleeding or vascular complications6. In patients receiving a VCD, we hypothesised that patients with US-guided femoral access would experience fewer access site complications than patients without US-guided femoral access. The present study aims to explore the association between US-guided femoral access and VCD use on bleeding and vascular complications in the prespecified VCD subgroups of the UNIVERSAL trial.
Methods
Study design and patients
The Routine Ultrasound Guidance for Vascular Access for Cardiac Procedures (UNIVERSAL) trial was a multicentre, open-label, investigator-driven, randomised clinical trial comparing US-guided femoral access versus no US for coronary procedures on a background of fluoroscopic landmarking for the primary composite endpoint of major bleeding and vascular complications (ClinicalTrials.gov: NCT03537118)6. The details of the design of the trial have been previously published7. Patients were eligible if referred for coronary angiogram or PCI with planned femoral access. Exclusion criteria were minimal: 1) age 18 years or younger, 2) ST-elevation myocardial infarction as the initial presentation, and 3) absence of a palpable femoral pulse. Eligible patients were randomised 1:1, stratified by planned VCD use, to femoral access with either US or no US. The subgroup analyses were performed by actual post-randomisation VCD use in patients randomised to US versus no US as specified in the protocol. Of note, US was used only to guide the femoral puncture and not to subsequently guide the VCD deployment.
The Population Health Research Institute, a joint institute of McMaster University and Hamilton Health Sciences, conducted the UNIVERSAL trial. The trial was approved by the ethics committee at each participating centre.
Clinical study outcomes
The primary outcome was the composite of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding or major vascular complications, including femoral artery pseudoaneurysm, arteriovenous fistula, retroperitoneal bleed, large haematoma more than 5 cm in diameter, or an ischaemic limb requiring intervention or surgery at 30 days.
A blinded investigator evaluated the presence of major bleeding or vascular complications at discharge. Furthermore, investigators blinded to allocation completed a comprehensive medical record review and telephone follow-up within 30 days of the index procedure.
Angiographic core laboratory evaluation
Femoral angiograms were reviewed by blinded research personnel in the angiographic core laboratory at the Hamilton General Hospital, Hamilton, Canada. We reported the location of the femoral bifurcation, the presence of common femoral artery calcification, the successful cannulation of the common femoral artery, active bleeding, and the presence of femoral artery dissection.
Statistical analysis
We presented continuous variables with normal distributions as means with standard deviations and compared groups using the Student’s t-test. We presented categorical variables as absolute frequencies and percentages and compared groups using the chi-square or Fisher’s exact test as appropriate. For the main outcomes, odds ratios (OR) with 95% confidence intervals (CI) were calculated. We calculated interactions between VCD subgroups and treatment allocation, using the Breslow-Day test of homogeneity, for the primary outcome, major bleeding alone, and vascular complications alone. We similarly analysed the different types of VCD. Two-tailed p-values <0.05 were considered statistically significant. We did not adjust for multiplicity. All analyses were performed using SAS version 9.4 (SAS Institute).
Results
Baseline characteristics
As demonstrated in Figure 1, 311 patients were allocated to US compared to 310 patients who were allocated to no US on a background of fluoroscopic guidance. No patients were lost to follow-up. A total of 170 of 311 (54.7%) patients in the US arm received a VCD, compared to 158 of 310 (51.0%) patients in the no US arm, for a total of 328 VCD patients.
The baseline characteristics were well balanced between subgroups when comparing US guidance to no US guidance, after stratification for VCD use (Table 1). In the VCD subgroup, the patients had a mean age of 70 years, 84 (25.6%) were female, and they had a mean body mass index of 29.4 kg/m2. The VCD subgroup had a comorbid population: 178 (54.2%) had a prior myocardial infarction, 170 (51.8%) had a previous PCI, 177 (54.0%) had prior coronary artery bypass surgery, 131 (39.9%) had diabetes mellitus, 43 (13.1%) had peripheral artery disease, 49 (14.9%) had atrial fibrillation, with similar rates of comorbidities in the no VCD group. Chronic total occlusion PCI was performed in 60 (18.3%) patients in the VCD subgroup.
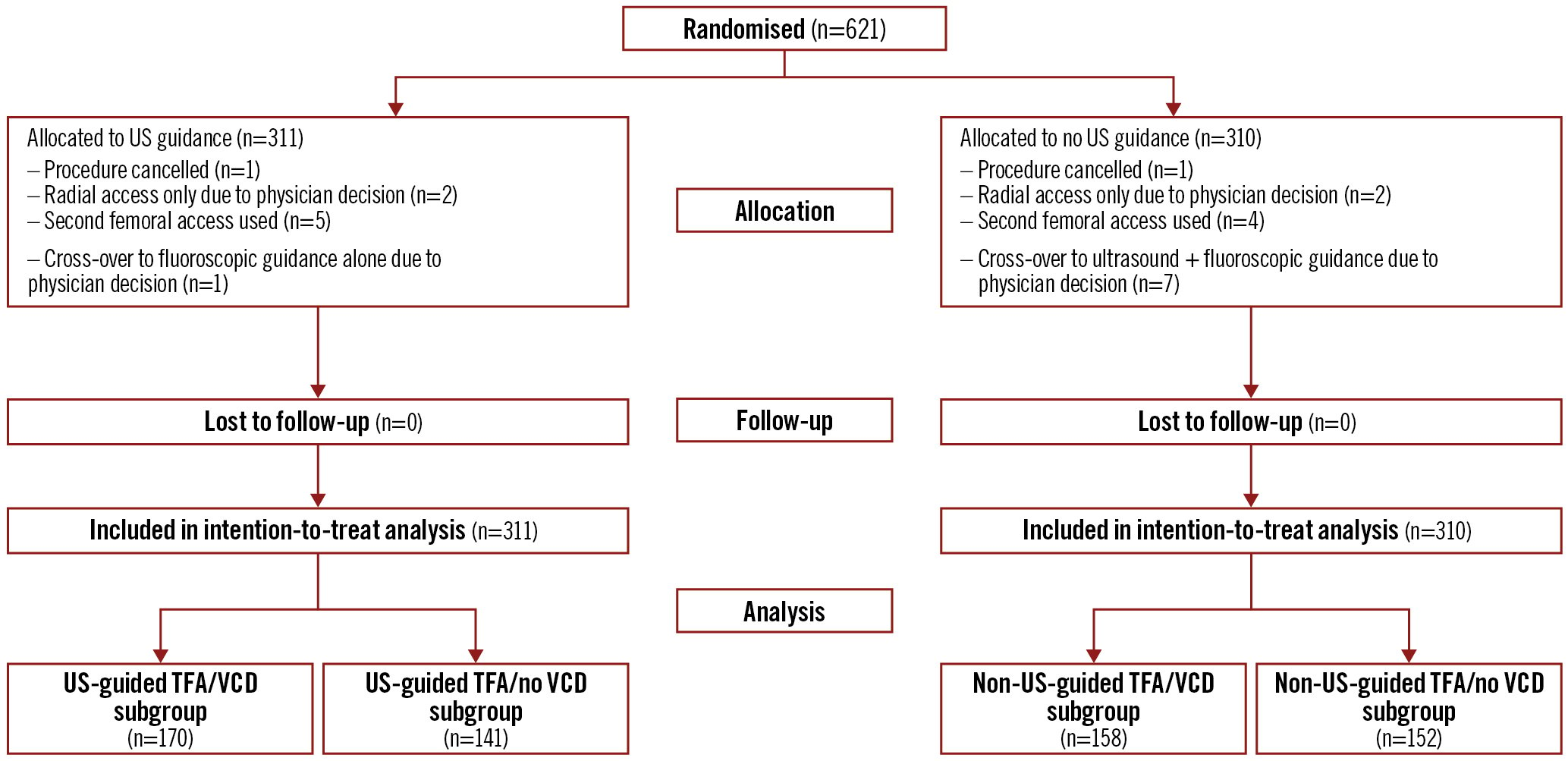
Figure 1. CONSORT diagram for the UNIVERSAL trial with vascular closure device subgroups. TFA: transfemoral access; US: ultrasound; VCD: vascular closure device
Table 1. Baseline characteristics.
| Variables | Vascular closure device use (n=328) |
p-value | No vascular closure device (n=293) |
p-value | ||
|---|---|---|---|---|---|---|
| US-guided (n=170) |
Non-US-guided (n=158) |
US-guided (n=141) |
Non-US-guided (n=152) |
|||
| Demographics and comorbidities | ||||||
| Age (years) | 69.89±10.14 | 69.63±10.40 | 0.82 | 71.15±10.14 | 71.71±10.25 | 0.64 |
| Female sex | 41 (24.1%) | 43 (27.2%) | 0.52 | 39 (27.7%) | 35 (23.0%) | 0.36 |
| BMI1 (kg/m2) | 29.91±6.01 | 28.79±6.35 | 0.10 | 28.99±6.08 | 28.90±6.60 | 0.90 |
| Hypertension | 144 (84.7%) | 141 (89.2%) | 0.22 | 120 (85.1%) | 133 (87.5%) | 0.55 |
| Dyslipidaemia | 147 (86.5%) | 140 (88.6%) | 0.56 | 126 (89.4%) | 142 (93.4%) | 0.21 |
| Diabetes | 74 (43.5%) | 57 (36.1%) | 0.17 | 59 (41.8%) | 71 (46.7%) | 0.40 |
| Current smoker | 27 (15.9%) | 30 (19.0%) | 0.46 | 13 (9.2%) | 22 (14.5%) | 0.17 |
| Previous myocardial infarction | 88 (51.8%) | 90 (57.0%) | 0.35 | 68 (48.2%) | 73 (48.0%) | 0.76 |
| Previous PCI | 87 (51.2%) | 83 (52.5%) | 0.81 | 53 (37.6%) | 55 (36.2%) | 0.80 |
| Previous CABG | 92 (54.1%) | 85 (53.8%) | 0.95 | 86 (61.0%) | 90 (59.2%) | 0.76 |
| Previous stroke/TIA | 15 (8.8%) | 13 (8.2%) | 0.85 | 10 (7.1%) | 20 (13.2%) | 0.09 |
| Peripheral vascular disease | 24 (14.1%) | 19 (12.0%) | 0.44 | 33 (23.4%) | 34 (22.4%) | 0.83 |
| Atrial fibrillation | 31 (18.2%) | 18 (11.4%) | 0.08 | 27 (19.2%) | 39 (25.7%) | 0.18 |
| Chronic kidney disease | 20 (11.8%) | 17 (10.8%) | 0.77 | 30 (21.3%) | 26 (17.1%) | 0.36 |
| PCI performed during procedure | 97 (57.1%) | 92 (58.2%) | 0.83 | 37 (26.2%) | 36 (23.7%) | 0.61 |
| Indication for procedure | ||||||
| Atypical chest pain | 20 (11.8%) | 15 (9.5%) | 0.51 | 17 (12.1%) | 31 (20.4%) | 0.05 |
| Stable angina | 54 (31.8%) | 33 (20.9%) | 0.03 | 34 (24.1%) | 35 (23.0%) | 0.83 |
| Silent ischaemia | 3 (1.8%) | 3 (1.9%) | 1.0 | 5 (3.6%) | 6 (4.0%) | 1.0 |
| Unstable angina | 21 (12.4%) | 11 (7.0%) | 0.10 | 16 (11.4%) | 7 (4.6%) | 0.03 |
| NSTEMI | 30 (17.7%) | 38 (24.1%) | 0.15 | 24 (17.0%) | 31 (20.4%) | 0.46 |
| Planned PCI | 14 (8.2%) | 9 (5.7%) | 0.37 | 6 (4.3%) | 3 (2.0%) | 0.32 |
| CTO PCI | 27 (15.9%) | 33 (20.9%) | 0.24 | 15 (10.6%) | 13 (8.6%) | 0.54 |
| Valvular assessment | 2 (1.2%) | 2 (1.3%) | 1.0 | 7 (5.0%) | 1 (0.7%) | 0.61 |
| Other | 11 (6.5%) | 18 (11.4%) | 0.12 | 25 (17.7%) | 33 (21.7%) | 0.46 |
| Medications at baseline | ||||||
| Aspirin | 153 (90.0%) | 143 (90.5%) | 0.88 | 105 (74.5%) | 119 (78.3%) | 0.44 |
| Plavix | 82 (48.2%) | 70 (44.3%) | 0.51 | 47 (33.3%) | 51 (33.6%) | 0.97 |
| Ticagrelor | 19 (11.2%) | 28 (17.7%) | 0.09 | 14 (9.9%) | 9 (5.9%) | 0.20 |
| Prasugrel | 0 (0.0%) | 0 (0.0%) | 1.0 | 0 (0.0%) | 0 (0.0%) | 1.0 |
| Warfarin | 6 (3.5%) | 1 (0.6%) | 0.12 | 7 (5.0%) | 10 (6.6%) | 0.55 |
| NOAC | 18 (10.6%) | 15 (9.5%) | 0.74 | 20 (14.2%) | 26 (17.1%) | 0.49 |
| Fondaparinux | 16 (9.4%) | 11 (7.0%) | 0.42 | 5 (3.6%) | 7 (4.6%) | 0.77 |
| Unfractionated heparin | 2 (1.2%) | 7 (4.4%) | 0.09 | 5 (3.6%) | 8 (5.3%) | 0.58 |
| Low-molecular-weight heparin | 2 (1.2%) | 0 (0.0%) | 0.50 | 5 (3.6%) | 1 (0.7%) | 0.11 |
| Bivalirudin | 1 (0.6%) | 0 (0.0%) | 1.0 | 0 (0.0%) | 0 (0.0%) | 1.0 |
| GP IIa/IIIb inhibitors | 0 (0.0%) | 0 (0.0%) | 1.0 | 0 (0.0%) | 0 (0.0%) | 1.0 |
| Anticoagulation during procedure | ||||||
| UFH use | 114 (67.1%) | 112 (70.9%) | 0.48 | 44 (31.2%) | 58 (38.2%) | 0.21 |
| Total dose of heparin (PCI procedure) | 8,078.1±3,151.7 | 7,443.5±2,837.7 | 0.06 | 7,494.4±3,396.0 | 7,705.6±2,615.8 | 0.55 |
| Final ACT | 295.73±110.76 | 293.76±102.72 | 0.87 | 243.92±94.64 | 259.44±98.69 | 0.17 |
| Data are expressed as mean±standard deviation (SD) or n (%). 12 patients had missing BMI values. ACT: activated clotting time; BMI: body mass index; CABG: coronary artery bypass graft; CTO: chronic total occlusion; GP: glycoprotein; NOAC: non-vitamin K antagonist oral anticoagulants; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack; UFH: unfractionated heparin; US: ultrasound | ||||||
Procedural characteristics and core laboratory data in VCD patients
A 6 Fr introducer was used for most accesses (n=246/332, 74.1%), and 141 of 172 (82.0%) US-guided accesses compared to 143 of 160 (89.4%) of non-US-guided accesses received an ANGIO-SEAL (Terumo) device (Table 2). Closure device failures according to access site were similar in both groups, with 16/172 (9.3%) failures in the US-guided subgroup and 11/160 (6.9%) failures in the non-US-guided subgroup (p=0.42) (Supplementary Table 1).
Successful cannulation of the common femoral artery (above the bifurcation and below the inferior epigastric artery) occurred in 148/169 (87.6%) of the US-guided femoral access sites compared to 141/156 (90.4%) of the non-US-guided femoral access sites (p=0.42) (Supplementary Table 2).
Table 2. Procedural characteristics in patients receiving a vascular closure device (by access site, as chronic total occlusion procedures may have had two access sites).
| Variables | US-guided (n=172) |
Non-US-guided (n=160) |
p-value | |
|---|---|---|---|---|
| Right-sided femoral access | 165 (95.9%) | 152 (95.0%) | 0.68 | |
| Introducer size | 5 Fr | 0 (0.0%) | 0 (0.0%) | 1.0 |
| 6 Fr | 127 (73.8%) | 119 (74.4%) | 0.91 | |
| 7 Fr | 37 (21.5%) | 37 (23.1%) | 0.72 | |
| 8 Fr | 7 (4.1%) | 2 (1.3%) | 0.17 | |
| Type of closure device | ANGIO-SEAL | 141 (82.0%) | 143 (89.4%) | 0.06 |
| ProGlide | 29 (16.9%) | 17 (10.6%) | 0.10 | |
| Other/missing | 2 (1.2%) | 0 (0.0%) | 0.50 | |
| Data are expressed as n (%). | ||||
Main outcomes
In patients who received a VCD, those who were randomised to US-guided femoral access compared to non-US-guided femoral access experienced a reduction in major bleeding or vascular complications (20/170 [11.8%] vs 37/158 [23.4%], OR 0.44, 95% CI: 0.23-0.82). In patients who did not receive a VCD, there was no difference between the US-guided and non-US-guided femoral access subgroups (20/141 [14.2%] vs 13/152 [8.6%], OR 1.76, 95% CI: 0.80-4.03). We observed significant interaction (interaction p=0.004) (Figure 2). More specifically, patients receiving an ANGIO-SEAL who were randomised to the US group experienced significantly fewer access site complications than patients who were randomised to the no US group (OR 0.33, 95% CI: 0.15-0.71). This effect was non-significant for the ProGlide (Abbott) VCD, with the caveat of wide confidence intervals (OR 0.74, 95% CI: 0.17-3.31). There was no significant interaction (interaction p=0.29).
When considering individual outcomes in patients who received a VCD, US-randomised patients had fewer major vascular complications than no US patients (7/170 [4.1%] vs 21/158 [13.3%], OR 0.28, 95% CI: 0.10-0.71). This beneficial effect of US was not observed in patients who did not receive a VCD (13/141 [9.2%] vs 8/152 [5.3%], OR 1.82, 95% CI: 0.68-5.25; interaction p=0.004). However, in patients who received a VCD, those randomised to US guidance did not experience a decrease in major bleeding as compared to those randomised to no US guidance (16/170 [9.4%] vs 24/158 [15.2%], OR 0.58, 95% CI: 0.28-1.19). Similarly, in patients who did not receive a VCD, those randomised to US-guided femoral access did not fare better than their non-US-guided counterparts (15/141 [10.6%] vs 9/152 [5.9%] OR 1.89, 95% CI: 0.74-5.07). There was, however, significant interaction (interaction p=0.034). Haematomas of more than 5 cm were approximately 3-fold more frequent in the VCD subgroup of patients randomised to no US compared to those randomised to US (4.1% vs 12.0%, OR 0.32, 95% CI: 0.11-0.81) (Supplementary Table 3).
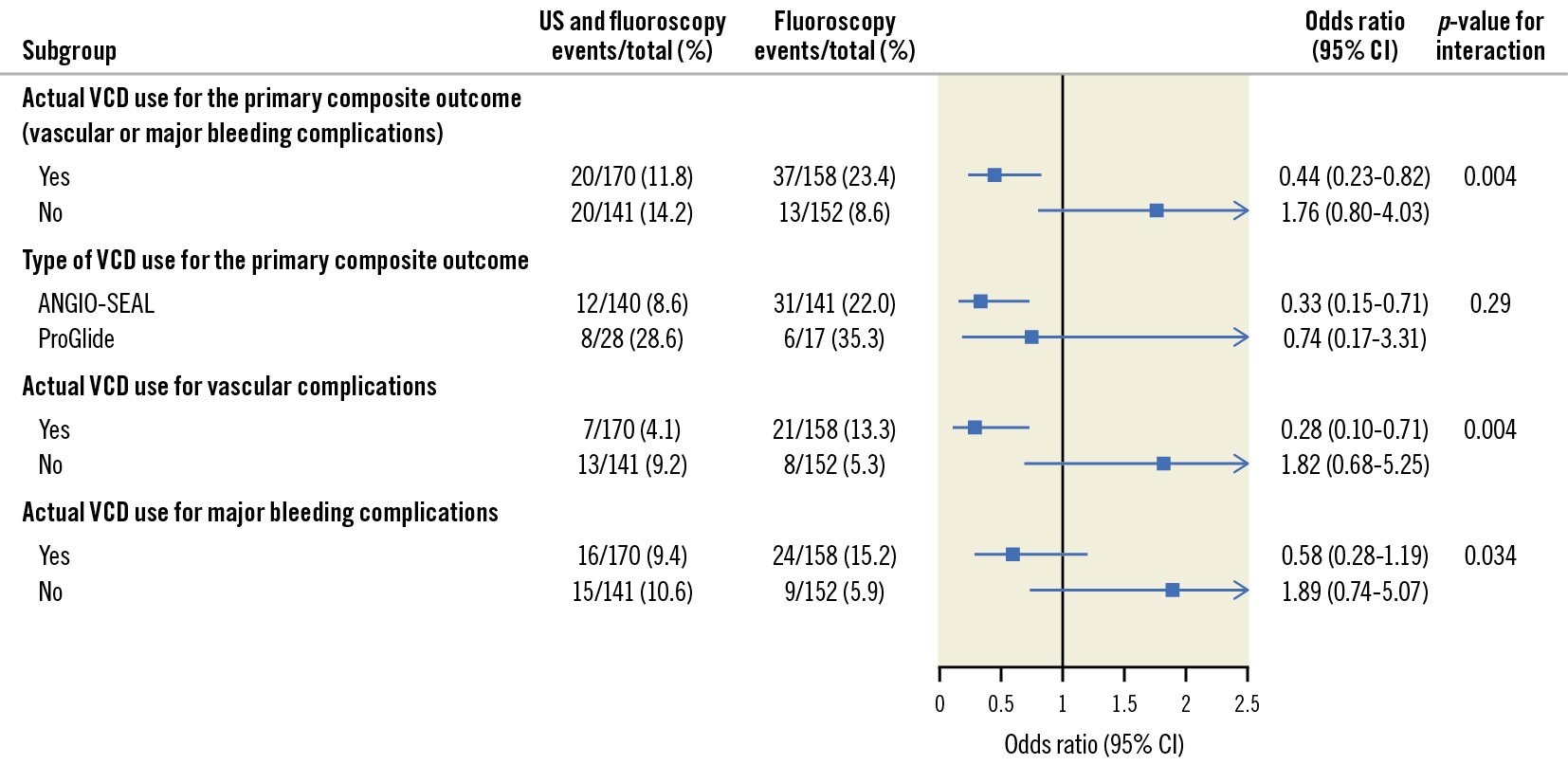
Figure 2. Main outcomes. CI: confidence interval; US: ultrasound; VCD: vascular closure device
Discussion
In patients who received a VCD, US- compared to non-US-guided femoral access reduced the composite of major bleeding or vascular complications in patients undergoing coronary angiography or PCI. This is likely the most important subgroup finding of the UNIVERSAL trial, as randomisation was stratified for intention to use a VCD. We also observed a reduction in major vascular complications with US in this subgroup but not a reduction in BARC 2, 3 or 5 major bleeding.
Importantly, we prespecified this subgroup and stratified the randomisation process according to planned VCD use. Before the trial, we believed that a significant interaction between US use and VCD existed, with benefit for US use. One explanation for the finding was that in the UNIVERSAL trial, patients randomised to US-guided femoral access had fewer attempts for arterial puncture (1.16 vs 1.43; p<0.001) and fewer venipunctures (3.1% vs 11.7%; p<0.001). These data are consistent with previous meta-analyses89. Reducing the number of arterial punctures is essential, as a VCD will only close one puncture site and will not mitigate venous bleeding. This hypothesis may be supported by our finding that large haematomas were more frequent in patients receiving VCD to close non-US-guided punctures compared to US-guided punctures. Conversely, manual compression may decrease bleeding from multiple arterial and venous punctures. US-guided femoral access allows the operator to avoid heavily calcified and diseased areas, allowing for safer and more efficacious deployment of a VCD10. However, we found that there was no difference in the rate of VCD failure between US- and non-US-guided femoral access. Previous randomised data demonstrated an increase in the efficacy of haemostasis with VCD use without a trade-off in safety, providing a strong rationale for the routine use of VCD over manual compression411. Our data strengthens this rationale by suggesting that VCD use can be even safer with US-guided femoral access and that operators should routinely use US-guided femoral access when a VCD closure strategy is planned.
While the UNIVERSAL trial results were neutral, the updated meta-analysis of 9 randomised controlled trials including 4,410 patients comparing US-guided femoral access to non-US-guided femoral access demonstrated a significant 42% relative risk reduction in vascular complications or major bleeding, favouring US guidance6. Therefore, we cannot assume that the benefit of US guidance only exists for patients receiving VCD.
In the ISAR-CLOSURE trial, 3,015 patients were randomly assigned to a VCD group, and 1,509 patients were assigned to manual compression. The primary endpoint occurred in 208 (6.9%) patients assigned to a VCD and 119 (7.9%) assigned to manual compression (p for non-inferiority <0.001)4. The notably higher event rate for patients receiving a VCD in the UNIVERSAL trial is likely multifactorial: 1) ISAR-CLOSURE patients only underwent diagnostic angiography while 262/621 (42.2%) UNIVERSAL patients underwent PCI; 2) ISAR-CLOSURE patients only had 6 Fr introducers while 121/621 (19.5%) UNIVERSAL patients had introducers larger than 6 Fr; 3) ISAR-CLOSURE only recruited elective diagnostic patients while 178/621 (28.7%) of UNIVERSAL patients had non-ST-segment elevation acute coronary syndromes; and 4) UNIVERSAL included BARC 2 bleeding while no equivalent event definition was used in the ISAR-CLOSURE trial. Of note, BARC 2 bleeding accounted for most of the events in UNIVERSAL.
We did not observe a significant difference in successful cannulation of the common femoral artery, consistent with the FAUST Trial12. One possible explanation is the effect of trainees transitioning through their learning curve, as half of the procedures were performed by interventional fellows. Another plausible explanation is that certain operators may have deliberately or inadvertently cannulated the femoral artery in a suboptimal location to avoid diseased or heavily calcified segments of the common femoral artery. Nevertheless, formal education and rigorous training are essential to developing and maintaining proper techniques.
We caution against comparisons of event rates between different closure devices in our study, as these were not randomised, and the choice may have been biased by operator and patient characteristics12. Nevertheless, the ProGlide VCD is typically associated with a higher learning curve than the ANGIO-SEAL, and the high rate of trainee participation in the UNIVERSAL trial may have impacted the outcomes13.
The benefit of US in femoral access, demonstrated by the present analysis and the meta-analysis, does not obviate the superiority of transradial access over transfemoral access. The randomised controlled 2x2 factorial SURF trial of US versus no US and transfemoral versus transradial access demonstrated that transradial access was superior to transfemoral access for the primary outcome of major bleeding, major adverse cardiovascular events comprising death, stroke, myocardial infarction, urgent target lesion revascularisation, or major vascular complications at 30 days. While US increased procedural efficiency, it did not reduce the primary outcome, even in the transfemoral subgroup14.
Limitations
The present study has several limitations. First, subgroup analyses should be considered hypothesis-generating. Second, as VCD use was a post-randomisation variable, US guidance may have biased the decision to use a VCD. However, as the patient randomisation was stratified by planned VCD use, baseline characteristics, procedural characteristics, and core laboratory findings were well balanced between the US-guided and non-US-guided subgroups receiving a VCD. Furthermore, even with potential selection bias, bailout to manual compression was a safe alternative, as demonstrated in our study. Third, the majority of the VCD used in the trial were ANGIO-SEAL devices, reducing the external validity of the results to all VCD. Fourth, the micropuncture technique was not used in the UNIVERSAL trial. However, it is plausible that using micropuncture needles mitigates the potentially deleterious effect of more inadvertent arterial punctures associated with no US use. Fifth, we did not provide a standardised definition for device failure to study investigators and caution against overinterpretation. Finally, as for the UNIVERSAL trial, the outcomes were primarily driven by BARC 2 bleeding, which is of less clinical importance than BARC 3 or 5 bleeding or vascular complications. However, a trial adequately powered for these outcomes would have required a substantial increase in the study sample size.
Conclusions
In patients receiving VCD after coronary procedures, US-guided compared to non-US-guided femoral access was associated with fewer major bleeding and vascular complications. US may be particularly beneficial when a vascular closure device is used.
Impact on daily practice
It is unknown whether ultrasound-guided femoral access reduces access site complications in the subgroup of patients receiving a vascular closure device. In patients who received a VCD, those randomised to US-guided femoral access compared to non-US-guided femoral access experienced a reduction in major bleeding or vascular complications (20/170 [11.8%] vs 37/158 [23.4%], OR 0.44, 95% CI: 0.23-0.82). In patients who did not receive a VCD, there was no difference between the US-guided and non-US-guided femoral access groups (20/141 [14.2%] vs 13/152 [8.6%], OR 1.76, 95% CI: 0.80-4.03; interaction p=0.004). Education and training of current and future interventional cardiologists with ultrasound for femoral access is needed.
Acknowledgements
The authors would like to acknowledge the Hamilton Health Sciences and Population Health Research Institute clinical and research personnel for their dedication to the UNIVERSAL trial.
Funding
Funding was provided by grants from the Hamilton Health Sciences Foundation and McMaster University, Division of Cardiology.
Conflict of interest statement
J. Velianou reports receiving grants or contracts, consulting fees, and payment or honoraria for lectures, presentations, speakers’ bureaus, or educational events from Edwards Lifesciences; and reports receiving payment for participation on a Data Safety Monitoring Board or advisory board from Edwards Lifesciences. M. Sibbald reports receiving payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Abbott and Philips. S.S. Jolly reports receiving grants or contracts from Boston Scientific; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Penumbra. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

