Abstract
Aims: To compare in a randomised trial the safety and efficacy of the FemoSeal vascular closure device (VCD) versus manual compression (MC) after femoral access coronary angiography (CAG).
Methods and results: In 13 months, 1,014 patients were included and 1,001 patients entered analysis. Median [interquartile range] closure time was 8.0 [6-10] minutes after MC versus 1.0 [1-1] minute (p<0.0001) for the FemoSeal VCD. Bed rest for one hour after the closure procedure was recommended in both groups. The primary endpoint of incidence of large groin haematoma was 6.7% in the MC group vs. 2.2% (p=0.002) in the FemoSeal group. The combined endpoint of 14-day adverse vascular events occurred in 1.0% in the MC group vs. 0.6% in the FemoSeal VCD group (p=0.7). Manual compression (OR 3.3, 95% CI: 1.5-7.2, p=0.002), female gender (OR 2.1, 95% CI: 1.1-3.9, p=0.018), and multiple punctures (OR 10.5, 95% CI: 3.2-34.3, p=0.001) were identified as independent predictors of adverse events and large haematomas.
Conclusions: Closure of femoral access after coronary angiography by the FemoSeal vascular closure device was safe, faster, and associated with significantly fewer in-hospital large haematomas as compared to closure by manual compression.
Introduction
Coronary angiography (CAG) is the standard procedure for invasive evaluation of coronary artery disease. The exact worldwide number of CAGs per year is unknown but is counted in millions. Although radial access for CAG is increasing in numbers, femoral artery access is still an extensively used access site for CAG. The principal safety concerns when performing CAG are complications related to femoral artery access, such as bleeding, groin haematoma, pseudoaneurysm, and stenosis or closure of the femoral artery1. Manual compression is the standard method for femoral access closure. Closure of femoral artery access after CAG using semi-automatic vascular closure devices (VCD) is increasingly utilised. In the United States, VCDs are used in an estimated 30% of coronary interventional procedures2. Comparisons of closure by VCDs versus MC have shown conflicting results regarding safety and efficacy. Some VCDs have been associated with less oozing1, faster ambulation and better comfort3. Groups which might benefit from closure by VCDs have been identified in subgroup analysis4 and registries5,6 but, due to varying definitions, no differences in access-site-related major adverse vascular events have been established in a randomised trial7 or in a systematic meta-analysis1.
FemoSeal™ (St. Jude Medical, St. Paul, MN, USA) is a sandwich-type, over-the-wire, semi-automatic closure device. The vessel is closed by an inner seal and an outer locking disc held together by a resorbable multifilament (Figure 1). The discs are made of a non-thrombosing, fully resorbable polymer. The non-thrombosing disc properties of the FemoSeal VCD might have advantages in comparison to the design of the market leading and somewhat similar Angio-Seal VCD (St. Jude Medical, St. Paul, MN, USA). In the Svenska Coronar Angiografi- och Angioplastik Registret (SCAAR) annual report from 20078, treatment of 1,058 patients by FemoSeal resulted in three (0.2%) large access-site bleedings and no other complications, being the VCD with the lowest complication rate. However, no statistical comparisons with other devices or with MC were performed. These promising registry findings warranted a randomised comparison to MC in order to evaluate if superior safety was achievable by use of the FemoSeal VCD.
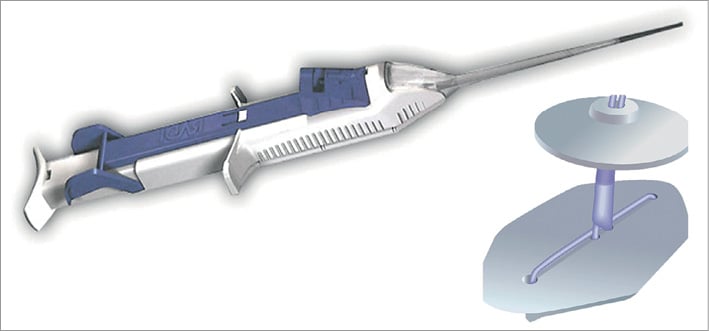
Figure 1. FemoSeal™. Vascular closure device (now St. Jude Medical, St. Paul, MN, USA). The semiautomatic delivery device and the sandwich-type seal discs shown. The inner seal disc and the outer locking disc are made of fully resorbable polymer containing no collagen or thrombosing agents. The discs are held together by a resorbable multifilament.
Methods
DESIGN
The CLOSure dEvices Used in everyday Practice (CLOSE-UP) study was an investigator-initiated, prospective, randomised, non-blinded single-centre trial conducted in a high-volume tertiary interventional heart centre in Western Denmark. The study was approved by the local medical ethics committee and registered at clinicaltrials.org (ClinicalTrials identifier: NCT01001663).
PATIENTS
Consecutive patients scheduled for elective diagnostic CAG at the Department of Cardiology, Aarhus University Hospital, Skejby, from September the 1st 2009 to September the 20th 2010 were included if eligible and if written informed consent could be obtained. Pain and discomfort experienced by included patients have been reported previously9.
The inclusion criteria were: eligibility for femoral access, age >18 years, the ability to provide written informed consent and use of a 6 Fr sheath. A patient could not be included if any wire was inserted in the coronary arteries. Patients with an expected lifespan of less than one year, patients with recent CAG (within one month) and patients with subsequent CAG within 14 days were also excluded. Other criteria for exclusion were: presence of groin haematoma before the closure procedure, known pseudoaneurysm at the femoral artery, sheath size other than 6 Fr, known stenosis of more than 50% in the femoral or iliac artery, INR above 3.0, platelet count less than 120×109 L–1, thrombolysis within 24 hours, terminal disease, pregnancy, systolic blood pressure of more than 200 mmHg and/or diastolic pressure exceeding 110 mmHg. Patients having femoral vein access during the same procedure were also excluded.
INCLUSION AND RANDOMISATION
Patients admitted for elective or subacute CAG were informed about potential participation in the study by cardiac care nurses in the ward prior to the procedure. Inclusion was offered at the catheterisation laboratory and informed written consent was obtained. Randomisation was performed at the end of the CAG, when it was concluded that no intracoronary assessment or treatment was to be done. Randomisation was performed 1:1 by telephone call to a voice prompt stand-alone computer-based system with block randomisation stratified by gender and diabetes.
PROCEDURE
Femoral artery access was obtained by direct puncture. CAG was performed according to best practice. Local guidelines were followed for administration of antithrombotic medication. The closure procedure was performed by the operator or by a trained nurse. Both high and low volume operators included patients. Operators and nurses were required to have performed ≥5 closure procedures using the FemoSeal VCD before performing the closure procedure in study patients. Ultrasound-guided access or femoral angiography prior to the closure procedure was not performed. Use of antithrombotic or anticoagulation therapy was registered.
CLOSURE BY FEMOSEAL
A wire was introduced in the 6 Fr sheath, the sheath removed, and the FemoSeal VCD inserted. After removing the wire and introducer, a sealing anchor plate was released inside the artery and withdrawn to seal the channel from the inside of the vessel. A locking plate was released outside the vessel wall by pulling the FemoSeal. By a single push button the two plates were moved towards each other to seal the arterial puncture site. The device was removed and the suture was cut under skin level to finalise the closure procedure.
CLOSURE BY MANUAL COMPRESSION
Closure by MC is a routine procedure at the study site. The 6 Fr sheath was removed immediately and MC was applied approximately 1.5 cm proximal to the puncture site by the operator or nurses trained in MC. Compression was continued for at least five minutes or until haemostasis. The subsequent use of sandbag compression was discouraged to avoid covering the access site, thereby risking unidentified bleeding and developing haematoma, and to improve patient comfort.
BED REST
One-hour bed rest was recommended for both treatments. During bed rest, the patient was allowed to raise his head to 45 degrees. Ward nurses were instructed to mobilise the patient after one hour of bed rest, if no additional bed rest was needed for clinical reasons.
COMPLICATIONS
Any complication was treated according to local practice. Mild oozing of blood after the closure procedure was treated with a plaster. A larger bleeding and evolving haematoma was treated by manual compression. In case of persistent or new onset pain after returning to the ward, the patient was examined by a medical doctor. If pseudoaneurysm formation, arteriovenous fistula, femoral stenosis, or retained closure material was suspected, an ultrasound examination was performed to confirm the diagnosis and to guide necessary therapy. Major bleeding was assessed clinically and necessary blood samples including haemoglobin values were obtained. Blood loss was treated according to local practice. In case of clinical signs of blood loss with minor or no demonstrable bleeding the patient was evaluated by a vascular surgeon and a computerised tomography scan performed to detect possible retroperitoneal haematoma.
ENDPOINTS
The primary endpoint was the in-hospital incidence of access-site haematoma >5 cm. The evaluation was performed immediately after the closure procedure and at discharge. Nurses in the wards performing the endpoint assessment were instructed to refrain from asking the patient about the precise closure method.
Secondary endpoints included 14-day major bleeding, retroperitoneal bleeding, pseudoaneurysm, arteriovenous fistula, infection, other complications necessitating surgery and the composite of these (major adverse vascular events; MAVE). Other endpoints were access-site haematoma >5 cm at 14 days, time to haemostasis, time to ambulation, device deployment failure, need for repeat manual compression after haemostasis was obtained, vasovagal response, and patients seeking medical assistance for access-site-related symptoms after discharge.
ENDPOINT DEFINITIONS
Haematoma >5 cm was defined as a palpable groin swelling measuring more than 5 cm at the longest diameter by use of a ruler. Major access-site bleeding was defined by evidence of access-site bleeding and clinical symptoms of new onset anaemia and clinical indication for treatment by blood components. Pseudoaneurysm and arteriovenous fistula had to be verified by ultrasound examination. Haemostasis was defined as cessation of bleeding; insignificant oozing manageable by a plaster was allowed. Device failure was defined as any technical failure of the device or any unsuccessful deployment of the VCD necessitating immediate MC. Vasovagal reaction was defined as sudden onset reversible nausea, pallor, vomiting and/or loss of consciousness and evidence of bradycardia and/or significant drop in blood pressure during or within five minutes after sheath removal. Patients seeking medical assistance from their general practitioner or hospital within 14 days after discharge were registered if the contact could in any way be related to closure procedure complications. Access-site infection was defined as any antibiotic treatment initiated due to suspicion of access-site infection.
14-DAY FOLLOW-UP QUESTIONNAIRE
Patients were asked to measure the haematoma, if present, at 14 days after the index procedure. All patients received detailed instructions and a ruler for self-assessment including instructions for obtaining help for the measurements. Patients were also asked to report any contact to the healthcare system within 14 days after discharge. If the questionnaire was not returned, the patient was contacted by telephone.
STATISTICAL ANALYSIS
The study was powered for the primary endpoint of in-hospital incidence of haematomas larger than 5 cm. Large haematomas were expected to occur in 11% of patients in the MC group and in 6% in the FemoSeal group. With an alpha of 5% and power of 80%, 423 patients were needed in each group. The analysis was performed on an intention-to-treat basis. Continuous variables are presented as mean±SD or median values [interquartile range] depending on the distribution. Differences in categorical variables were analysed using the Fisher’s exact test or chi-square test, and continuous variables using the Student’s t-test when following a Gaussian distribution. Tests of significance were two-tailed. The Mann-Whitney U test was used if data were non-Gaussian-distributed despite log transformation. The level of significance was 5%. Independent predictors of in-hospital large haematoma and 14-day access-site-related MAVE were identified by a multivariable logistic regression model with direct entry of all of the following established clinical predictors of complications: study treatment, gender, age, hypertension, multiple punctures, prior revascularisation, peripheral arterial disease, aspirin treatment, clopidogrel treatment, and vitamin K antagonist treatment. All analyses were performed using STATA 10.1 (StataCorp, College Station, TX, USA).
STUDY CONDUCT
The steering committee consisted of NRH, BS, MS, MM, SDK and JFL. The steering committee was responsible for the conduct and safety of the study, all members had full access to the study data and all agreed on analysis and publication. The adverse endpoints were adjudicated by an independent endpoint committee consisting of Jakob Thorsted Soerensen, MD, PhD, and Carsten Stengaard, MD, both from the Dept. of Internal Medicine and Cardiology at Aarhus University Hospital. All endpoints were adjudicated according to protocol endpoint definitions.
Results
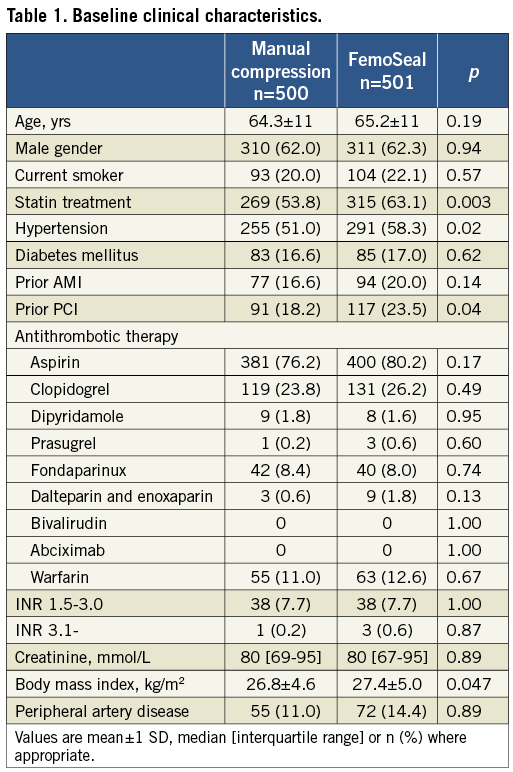
From September 2009 to September 2010, 1,014 patients were included in the study. Thirteen patients were excluded due to exclusion criteria as outlined in the flow chart (Figure 2) leaving 1,001 patients with complete in-hospital follow-up. A total of 501 patients underwent closure by FemoSeal VCD and 500 patients by MC. Fourteen-day follow-up was available in 483 (96.4%) patients in the FemoSeal group and in 481 (96.2%) patients in the MC group. No patients lost to follow-up died during the follow-up period. Baseline characteristics (Table 1) were balanced except that more patients in the FemoSeal group were treated for hypercholesterolaemia, hypertension, more often had a history of prior PCI, and had a higher body mass index.
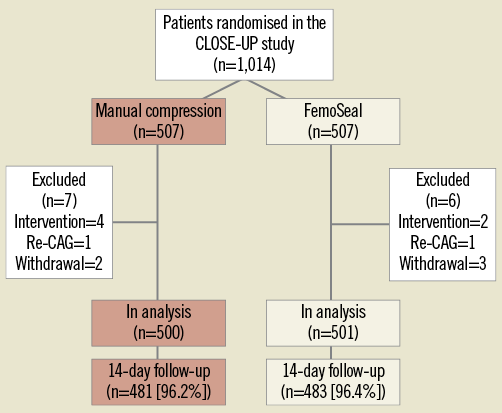
Figure 2. Flow chart. Flow chart of the randomised CLOSE-UP study and description of patients excluded from analysis.
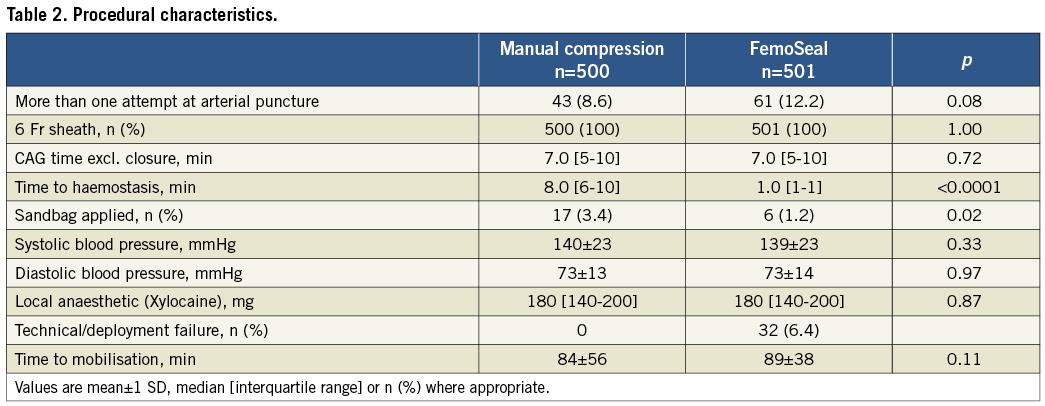
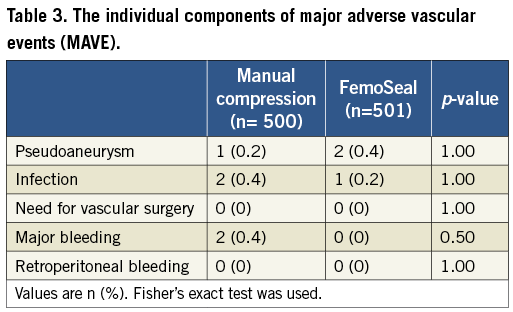
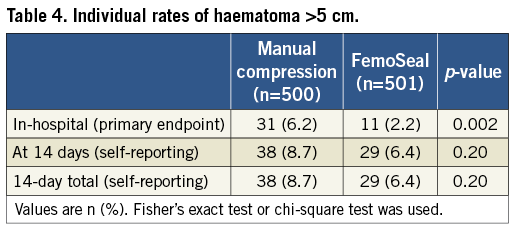
The primary endpoint of incidence of in-hospital groin haematoma exceeding 5 cm was 6.7% in the MC group versus 2.2% (p=0.002) in the FemoSeal group (Figure 3). Procedural data are shown in Table 2. Median [interquartile range] closure time was 8.0 [6-10] minutes after MC versus 1.0 [1-1] minute (p<0.0001) for FemoSeal. Duration of bed rest was 84±56 minutes for MC vs. 89±38 minutes (p=0.11) for FemoSeal. One patient in each group died during follow-up. The combined endpoint of access-site MAVE occurred in 1.0% in the MC group vs. 0.6% in the FemoSeal group (p=0.7). Individual results of access-site MAVE are shown in Table 3. FemoSeal device deployment failure was observed in 6.4% of cases and caused large haematomas in 36% of these cases but no major adverse events. Vasovagal response occurred during or immediately after closure in 0.8% in the MC group vs. 0.2% of cases in the FemoSeal group (p=0.18). New onset MC was instated in 8.8% vs. 11.4% (p=0.18) of cases for MC and FemoSeal, respectively. From discharge to 14 days post index procedure, 3.8% closed by MC vs. 4.2% (p=0.82) closed by FemoSeal sought medical assistance for possible or definite closure-related problems. At 14 days, no difference in self-measured large haematomas was detected (Table 4). Manual compression (OR 3.3, 95% CI: 1.5-7.2, p=0.002), female gender (OR 2.1, 95% CI: 1.1-3.9, p=0.018), and multiple punctures (OR 10.5, 95% CI: 3.2-34.3, p=0.001) were identified as independent predictors of the combined endpoint of in-hospital haematomas >5 cm and 14-day MAVE by multivariate logistic regression.
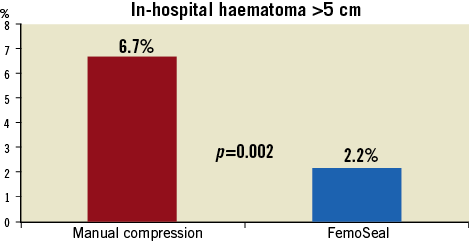
Figure 3. Primary endpoint. The primary endpoint: incidence of in-hospital groin haematomas larger than 5 cm. Patients treated with the FemoSeal vascular closure device (blue bar) had significantly fewer in-hospital large haematomas as compared to patients treated with manual compression (red bar).
Discussion
This is the largest randomised comparison of MC versus a VCD for closure after femoral access CAG reported so far. Our major finding is that closure by FemoSeal VCD was associated with significantly fewer large haematomas as compared to closure by MC. The incidence of MAVE was low and with no difference between the two groups.
GROIN HAEMATOMAS
The incidence of in-hospital groin haematomas >5 cm was reduced by an absolute 4.5% by FemoSeal VCD versus MC. In a systematic meta-analysis, the mean incidence of groin haematomas was 4.2% after VCD versus 5.7% after MC in patients examined by CAG1. The greater difference between VCD and MC in our study may be explained by different endpoint definitions, patient subsets, and use of different VCDs. The slightly higher haematoma rate in CLOSE-UP compared to the meta-analysis findings may be explained by inclusion of patients with multiple punctures which was shown to affect endpoint rates. The systematic assessment of haematoma size in both the catheterisation laboratory and in the ward may also have contributed to a more sensitive detection of haematomas.
Large groin haematomas are rarely a major vascular event causing deterioration of the patient´s circulation. However, large haematomas are a daily concern and patients may experience pain and discomfort from haematomas and from reiterated manual compression or device compression. Fever due to resorption of haematoma may be difficult to differentiate from other causes and leads to unnecessary antibiotic treatment. Also, large haematomas are likely to be associated with increased hospital costs. In the CLOSE-UP study, extended manual compression was applied in nearly all cases of in-hospital large groin haematomas, but additional diagnostics were only performed in a few cases. Importantly, the difference in large haematomas in CLOSE-UP was not associated with an increased risk of MAVE, and MC was found to be associated with less immediate pain and discomfort compared to closure by FemoSeal VCD with no difference after leaving the catheterisation laboratory9. The similar rates of repeat MC, despite differences in haematomas, indicate that superficial bleedings may contribute to minor events.
Late groin haematoma results at 14 days were not included in the primary endpoint due to difficulties in correct out-of-hospital measuring of the haematomas10. Our finding, in both groups, of an increasing rate of large haematomas at 14-day follow-up may reflect the spreading of minor subcutaneous bleedings as well as late bleedings in both study groups. After discharge, no patients sought medical assistance for new onset haematoma or bleedings, indicating that the increased number of haematomas at 14 days may be due to the downward migration of haematomas that were initially <5 cm.
MANUAL COMPRESSION
The optimal technique for manual compression is yet to be defined. The optimal duration of manual compression after the procedure has not been investigated. The definition applied in CLOSE-UP led to a large variation in the length of the procedure. The median compression time of eight minutes might have been too short, increasing the risk of subsequent bleeding, though this relation was not established. The use of sandbag was discouraged due to reports of patients having major groin complications evolving hidden under the bag. No advantage of sandbags has been established. The duration of bed rest after MC has been reduced substantially in recent years, and one-hour bed rest after CAG has been shown to be safe for standard care11.
SAFETY
MAVEs were rare in both groups, which may in part reflect a low-risk population and the fact that only patients without evidence of haematoma before the closure procedure could be randomised. Before this trial, limited evidence characterising the FemoSeal VCD was available. A small series with use of FemoSeal after 7 Fr guiding catheter PCI has indicated a favourable safety profile12. Our finding of no need for blood transfusion after FemoSeal closure in the CLOSE-UP study is in line with the 2007 SCAAR registry findings of a major access-site bleeding rate of 0.2%. In CLOSE-UP, as in previous studies, pseudoaneurysms were present but rare in both groups. Retroperitoneal haematoma13 and femoral artery dissection which have been reported with another VCD14 did not occur in the CLOSE-UP study.
The short time to haemostasis, low rate of large haematomas, and the absence of major bleeding complications in the FemoSeal group in CLOSE-UP might be attributed to the concept of a thin but rather large diameter inner sealing of the artery. Closure by VCD has been associated with increased risk of pseudoaneurysms15. Two patients (0.4%) had pseudoaneurysm formation following the closure procedure, and in both cases multiple punctures were performed to access the artery indicating an alternative explanation for these pseudoaneurysms. The fully absorbable sealing and locking discs may ensure low risk of 1) stenosis, 2) infection, and 3) long-term discomfort. The CLOSE-UP study was not designed to detect long-term effects of this closure method, but other VCD studies have shown good long-term results16.
DEPLOYMENT FAILURE
Deployment failure of VCDs is associated with an increased risk of adverse complications17. A total deployment failure of 6.4% was recorded in CLOSE-UP, similar to results obtained with DUETT (VasoSeal; Datascope Corp., Montvale, NJ, USA) and StarClose (Abbott Vascular, Santa Clara, CA, USA)3,18, and is higher than what has been reported for Perclose (Abbott Vascular) and Angio-Seal VCDs3,17,19,20 though with different definitions of deployment failure. In CLOSE-UP, deployment failure was also recorded in case of immediate profuse bleeding despite apparently normal deployment of the FemoSeal VCD. Deployment failure was associated with increased risk of large haematomas but, importantly, no MAVE occurred in these cases. Though endpoint rates were very low after FemoSeal VCD, the use of routine femoral angiography might have prevented some procedural problems. The two pseudoaneurysms found in the FemoSeal VCD group were likely associated with multiple punctures in both cases.
WORKFLOW IMPLICATIONS
The substantial reduction in time to haemostasis by FemoSeal is comparable to results achieved by other closure devices18,21. The duration of the closure procedure was also more predictable in the FemoSeal group compared to the MC group as judged by the difference in the range of time to haemostasis. In the busy or low-staffed catheterisation laboratory these efficacy parameters might be an important factor in favour of VCDs. Also, in laboratories with limited front-room space the use of VCDs may help to increase patient turnover. Similar needs for safe workflow optimisation are also being addressed for femoral access procedures with large profile tools22.
Same day discharge after CAG or PCI is feasible in selected patients with overnight stay needed in 20% of cases after elective PCI closed by MC23. During the inclusion period of CLOSE-UP, many patients were treated in the evenings and overnight stays were offered liberally on a convenience basis. Thus a time to discharge analysis was not possible. It is likely that a reduced rate of in-hospital access-site complications could reduce the need for overnight stay, thereby lowering costs23.
Limitations
A limitation applicable to this and other VCD studies is the lack of blinding. Unblinded in-hospital endpoint assessment was performed by multiple nurses and doctors, making the assessment less prone to personal bias. Closure by MC or VCDs is a delicate procedure and bias towards one of the treatments might have influenced conduct of the procedure. It is well known that there is a learning curve using VCDs, although MC is also a demanding procedure. FemoSeal was introduced in our catheterisation laboratory a few weeks before the start of the study. This might have led to underestimation of the true safety and efficacy of FemoSeal. Our limited follow-up time of 14 days might have increased the risk of missing later complications. The patient self-measurements of haematoma after 14 days using the ruler provided were undoubtedly associated with considerable variance. MC was performed according to local practice with immediate sheath removal, no use of sandbag and one hour of bed rest11. Since slight increases in complication rates are seen in some comparisons of late and early ambulation24, a longer bed rest after MC might have reduced the incidence of vascular events at the expense of less patient comfort25,26. Our conclusions are not necessarily applicable to patients having PCI as more intensive antithrombotic treatment is used during and after PCI.
Conclusion
Closure of femoral access after coronary angiography by the FemoSeal semiautomatic vascular closure device was associated with significantly fewer in-hospital large haematomas and was faster as compared to closure by manual compression. The risk of major adverse vascular events was similar for closure by manual compression and FemoSeal.
| Impact on daily practice Coronary angiography by femoral access with subsequent closure by manual compression or FemoSeal vascular closure device may be performed with very low risk of adverse vascular complications. The advantages of using FemoSeal VCD over manual compression are faster closure procedures and lower risk of large superficial hematoma. |
Acknowledgements
Thanks to the ward nurses and nurses at the catheterisation laboratory, Department of Cardiology, Aarhus University Hospital, Skejby, Denmark for their huge efforts in informing and including patients, doing measurements, and recording clinical data. Thanks to study secretaries Helle Bargsteen and Marianne Signa Dyrby for invaluable logistic support.
Funding
The CLOSE-UP study was supported by an unrestricted research grant by the Danish distributor of FemoSeal; Vingmed, Roskilde, Denmark. The sponsor had no influence on any aspect of the study. The study was planned and initiated when the FemoSeal VCD was produced by Radi Medical, Uppsala, Sweden. During the study period, Radi Medical was acquired by St. Jude Medical, St. Paul, MN, USA.
Conflict of interest statement
N. Holm has received speaker fees, travel grants and unrestricted research grants from St. Jude Medical. E.H. Christiansen has received unrestricted research grants from St. Jude Medical. J. Lassen has received unrestricted research grants from St. Jude Medical. The other authors have no conflicts of interest to declare.

