Abstract
Aims: To test the safety of immediate mobilisation of patients undergoing coronary angiography and percutaneous coronary intervention (PCI) closed with Angio-Seal™ –a femoral vascular closure device.
Methods and results: First, a randomised controlled trial of immediate mobilisation vs. delayed ambulation was performed followed by a prospective validation registry to test the obtained results in a real-world situation. The randomised trial comprised 300 patients; the validation registry comprised 1,097 patients. Primary endpoints were complications defined as: small haematoma <5cm and/or minor bleeding/oozing from the puncture site, haematomas ≥5 cm, bleeding needing transfusion, bleeding needing surgical attention, pseudoaneurysm and vasovagal reaction. In the randomised trial, overall complications were similar in both groups (16.0%vs.18.8%; p=0.53). Small haematomas/small bleedings/oozing were the most frequent (12.2% vs.15.3; p=0.44). There were no bleedings needing transfusion or surgical attention, and no pseudoaneurysms occurred. The prospective registry showed similar results. In the standard-care cohort, complications were similar to those in the implementation cohort (9.6% vs.11.3%; p=0.41), mainly consisting of small haematomas/minor bleedings/oozing (6.1% vs.7.3%; p=0.49). No bleedings needed transfusion or surgical attention. Pseudoaneurysms occurred in 1 (0.34%) vs. 3 (0.37%; p=0.94) and vasovagal reactions in three (1.0%) vs. four (0.5%; p=0.33) patients. It was possible to mobilise 87% of patients in the implementation cohort.
Conclusions: In patients undergoing coronary angiography or PCI, the use of immediate mobilisation after Angio-Seal™ deployment is safe. With routine use of a femoral vascular closure device, approximately 87% of patients are suitable for immediate mobilisation.
Introduction
The introduction of vascular closure devices (VCDs) has improved and simplified femoral access site management in patients undergoing cardiac catheterisation and percutaneous coronary intervention (PCI). VCDs and manual compression have equal complication rates1-4. The efficacy and safety of some of these devices with respect to early ambulation have been evaluated in small randomised trials and single-centre studies5-7. Compared with manual compression, VCDs improve patient satisfaction, reduce time to both haemostasis and ambulation and enable earlier discharge5,7-10. Accordingly, the current standard care in many catheterisation laboratories consists of deploying a femoral VCD after the intervention, followed by the patients being in supine position for 3-4 hours before ambulation or discharge. However, it could be hypothesised that the immediate mobilisation of these patients after the procedure would improve patient satisfaction, reduce resources for post-procedural care, and allow faster patient turnover by reducing the time to transfer or discharge.
To test the hypothesis that immediate mobilisation after use of a femoral VCD is safe in terms of local and systemic complications and bleeding, we performed a randomised clinical trial followed by a prospective real-world registry in patients undergoing coronary angiography or PCI via the femoral access to evaluate safety and efficacy of the Angio-Seal™ femoral VCD (St. Jude Medical, St. Paul, MN, USA).
Methods
Study design
To test the efficacy and safety of a femoral VCD with respect to immediate post-procedure ambulation, two studies were undertaken: first, a randomised controlled trial of immediate mobilisation vs. delayed ambulation after deployment of the Angio-Seal™ femoral VCD; second, a prospective validation registry to test the obtained results in a real-world situation. The studies were conducted at the University Hospital Basel, Basel, Switzerland and Gentofte University Hospital, Hellerup, Denmark which have close scientific collaboration11. All patients signed an informed consent form.
Study population
Randomised trial
We screened 485 patients for the randomised trial, of whom 185 (38%) were excluded for various reasons. To be mobilised immediately the procedure had to be uncomplicated, there had to be no puncture complications and the VCD had to be deployed without complications in a patient who did not receive oral anticoagulant therapy. There were 55 out of the 485 screened patients equivalent to 11% who did not meet these criteria: admission to coronary care unit with inability to ambulate immediately n=15, oral anti-coagulant therapy n=13, puncture complication n=11, unstable patient n=6, device deployment complications n=5, several punctures n=2, long complicated intervention n=2, and device failure n=1. An additional 140 (29%) were excluded for other reasons: concomitant right heart catheterisation n=32, venous puncture only for treatment of persistent foramen ovale n=20, patient refusal n=19, logistic reasons n=14, cancellation of procedure n=10, patient does not understand German n=9, patient mobility problems n=4, peripheral arterial disease n=3, severe aortic stenosis/insufficiency n=3, hypertension n=2, hypotension n=1, venous sheath n=1, eligible but not randomised for unknown reasons n=9, and reason unknown n=3. The remaining 300 patients were randomised to either immediate mobilisation (n=144, 48%) vs. delayed ambulation (n=156, 52%) after performance of a coronary angiography alone or a coronary angiography followed by PCI. The delayed ambulation strategy consisted of four hours of bed rest after the deployment of the femoral VCD with a tight bandage to compress the puncture site, and mobilisation of the patient afterwards. The immediate mobilisation strategy consisted of patients standing up and walking out of the catheterisation laboratory immediately after the procedure.
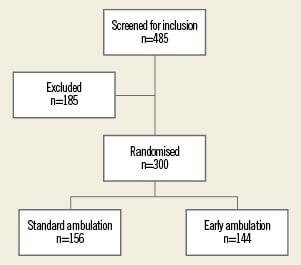
Figure 1. The flow of patients in the randomised trial.
Prospective validation registry
To evaluate the implementation of an immediate ambulation strategy for a large all-comer population of patients treated with the Angio-Seal™ device, a prospective validation registry was performed (n=1,097). Of all patients undergoing coronary angiography alone or combined with a PCI and receiving a femoral VCD, outcome was assessed to evaluate the effect of the change in procedure guidelines. The validation registry consisted of three phases: 1)standard-care phase, i.e., bed rest for three hours after procedure; 2) feasibility phase, i.e., immediate ambulation in coronary angiography patients but not in PCI patients; 3) implementation phase, i.e., immediate ambulation in all (coronary angiography and PCI) patients. Phase 1 consisted of 293 patients (standard-care cohort, 27%). Phases 2 and 3 were combined for outcome analysis (implementation cohort, 73%).
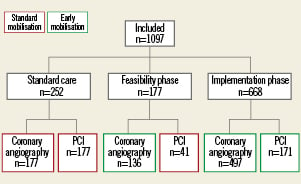
Figure 2. The flow of patients in the validation registry.
Outcomes
In both studies the primary endpoint was defined as the occurrence of one of the following: 1) haematomas <5 cm in diameter with either minimal bleeding or oozing from the puncture site, 2) haematomas ≥5 cm in diameter, 3) bleeding necessitating blood transfusion, 4) bleeding necessitating surgical attention, 5) pseudoaneurysm formation, and 6) vasovagal reaction. In the randomised controlled trial, complications were registered immediately in the catheterisation laboratory, after four hours, and before discharge from the ward; additionally, patients were followed-up by telephone after six months.
In the validation registry, complications were assessed in the catheterisation laboratory and before discharge from the ward. In this registry, patient satisfaction in relation to bed rest and mobilisation in the catheterisation laboratory and on the ward was registered using a standard VAS score. Patients were asked to evaluate three situations:
–To what extent did you feel discomfort when transferred to the bed in the catheterisation laboratory?
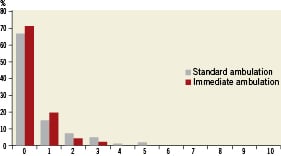
Figure 3. Answer to “To what extent did you feel discomfort when transferred in the catheterisation laboratory?”
–To what extent did you feel discomfort during the following observation period?
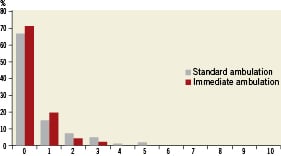
Figure 4. Answer to “To what extent did you feel discomfort during the following observation period?”
–To what extent did you feel discomfort in the first hour after ambulation?
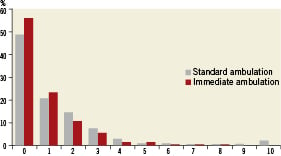
Figure 5. Answer to “To what extent did you feel discomfort in the first hour after ambulation?”
Patients were asked to score each situation on a 0-10 scale with0 being no discomfort and 10 being very uncomfortable.
Statistical analysis
For the randomised trial, no formal sample size calculation was performed as no accordant data were available in the literature. It was hypothesised that a cohort of 300 patients would be sufficient to have adequate power to show clinically meaningful differences.
Discrete data are presented as frequencies and percentages. In both studies, differences in baseline characteristics and outcome between the randomised groups were evaluated using Pearson χ2-test. In the validation registry, to test for differences between the standard and early-ambulation patient satisfaction, we used the nonparametric Mann Whitney U-test for differences in scores between the groups. Logistic regression analysis was done to examine independent predictors of outcome (all complications and complications other than small bleedings). Age, sex, diabetes, hypertension, type of procedure and immediate ambulation were included in the models. All hypothesis tests had a 0.05 significance level. All tests were two-sided. All analyses were performed with SAS statistical software package version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient population
Randomised trial
In the 156 patients in the delayed ambulation group, 93 (60%) coronary angiographies and 63 (40%) PCI procedures were performed; in the 144 patients in the immediate mobilisation group, 91 (63%) coronary angiographies and 53 (37%) PCI procedures were performed (p=0.52).
Validation registry
In the standard-care cohort (n=293), 177 (60%) coronary angiographies and 116 (40%) PCI procedures were performed; in the implementation cohort (n=804), 633 (79%) coronary angiographies and 171 (21%) PCI procedures were performed (p<0.0001). In the implementation cohort there were 108 (13%) patients who were not able to be mobilised early. Of these patients, 71 (66%) were coronary angiographies and the rest PCIs.
Baseline characteristics
Randomised trial
Baseline characteristics were largely similar between the randomised groups (Table 1). However, there were more men in the immediate mobilisation than in the delayed ambulation group (82 vs. 72%, p=0.05), and they had more peripheral artery disease (49% vs. 38%; p=0.04).
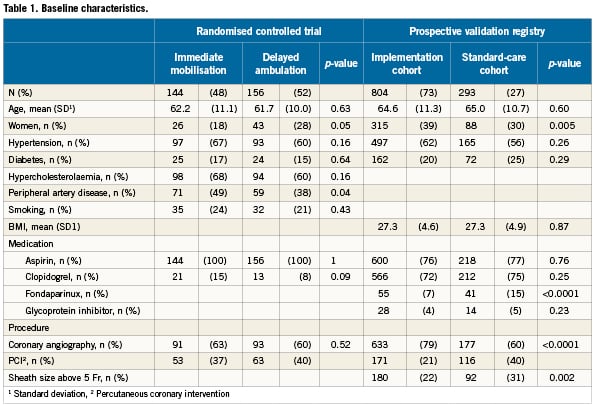
Validation registry
The number of procedures in each group differed by definition because only coronary angiography patients were included in the feasibility phase of the registry, whereas PCI patients were still treated according to the standard protocol. There were more women in the implementation cohort than in the standard-care cohort (39% vs. 30%; p=0.005). More patients in the standard-care cohort than in the implementation cohort received fondaparinux (15% vs. 9%; p=<0.0001) and had a sheath size over 5 Fr (31% vs. 22%; p=0.002).
Outcome
Randomised trial
Complications were similar in both randomised groups (16.0% vs. 18.8%; p=0.53; Table 2). The main component was small haematomas, minor bleedings and oozing, which were similar in both groups (12.2% vs. 15.3; p=0.44). There were no bleedings needing transfusion or surgical attention, and no pseudoaneurysms occurred.
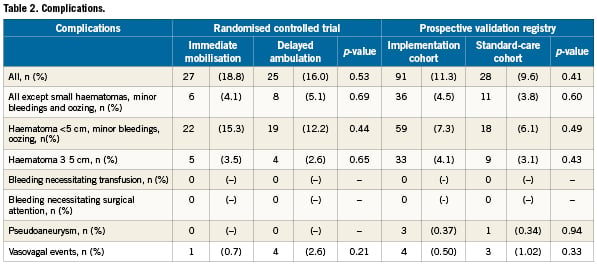
Validation registry
The prospective registry showed similar results. In the standard-care cohort, complications were similar to those of the implementation cohort (9.6% vs. 11.3%; p=0.41), mainly consisting of small bleedings (6.1% vs. 7.3%; p=0.49). Pseudoaneurysms occurred in one (0.34%) vs. three (0.37%) (p=0.94) and vasovagal reactions in three (1.0%) vs. four (0.5%) (p=0.33) patients. No bleedings needed transfusion or surgical attention. Of the 108 not immediately mobilised in the immediate mobilisation period complications were seen in 26 (24%), and of these 16 (15%) were small haematomas, minor bleedings and oozing and 10 (9%) were haematomas larger than 5cm and one (1%) had a vasovagal event. If the complications were seen before mobilisation the patients were not mobilised immediately. Other reasons for not immediately mobilising were: venous sheath n=5, puncture problems n=4, abciximab infusion n=3, elevated INR n=3, unstable patient n=3, groin pain n=1, low blood sugar levels n=1, patient refusing n=1, overweight n=1, anaphylactic reaction n=1.
For patient satisfaction we found no difference in the scores between the groups when looking at transferral in the catheterisation laboratory and the first hour after ambulation. However, there was a highly significant difference between the groups for the discomfort during bed rest, favouring the immediate ambulation group (p<0.001).
Combined analysis
In a logistic regression analysis of all patients from both studies (n=1,397), we identified predictors of any complication and predictors of complications other than small haematomas, minor bleedings and oozing (Table 3). For any complication, type of procedure (coronary angiography vs. PCI) was highly significant with an odds ratio (OR) of 3.75 (95% confidence interval [CI]: 2.66-5.27, p<0.0001) when the procedure was a PCI compared with a coronary angiography. The OR for immediate mobilisation was 1.31 (95% CI: 0.91-1.88, p=0.15) compared with delayed ambulation.
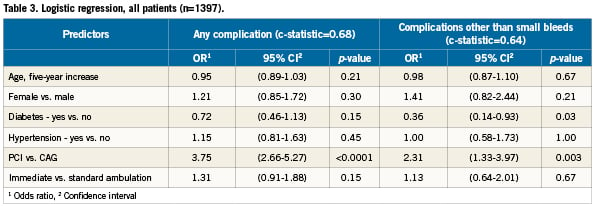
In complications other than small haematomas, minor bleedings and oozing, the only significant predictors were type of procedure with an OR of 2.31 (95% CI: 1.34-3.97, p=0.003) when performing PCI compared with CAG and diabetes with an OR of 0.36 (95% CI: 0.14-0.93, p=0.03). The models showed reasonable discrimination with a c-statistics of 0.68 and 0.64 respectively. The OR for immediate mobilisation was 1.13 (95% CI: 0.64-2.01, p=0.67) compared with that of delayed ambulation.
There are differences between the two groups in the prospective validation registry, and they are very closely linked to the differences in the number of PCIs in the two groups. When doing the logistic regression analysis with these parameters they do not add more information than what is added by type of procedure.
Discussion
Immediate mobilisation after implantation of the Angio-Seal™ femoral VCD is safe. Furthermore, it is possible to safely implement a strategy of immediate mobilisation with a femoral VCD in a large real-world population after coronary angiography and PCI. We showed that an immediate mobilisation strategy has a neutral to positive influence on the patient’s post-procedure experience. In addition, the nursing staff benefit from having fewer check tasks associated with bed-resting patients.
Several studies have shown manual compression and VCDs to be equally good in terms of complications1-3,12. Complication rates after implantation of femoral VCD are reported to be around 3-5% in randomised clinical trials12-15 and registry studies1-3,16,17. This is similar to the present findings as these reported complications correspond to the “complications except small haematomas, minor bleedings and oozing” in our study. Several studies have shown reduced time to haemostasis and ambulation using VCD versus mechanical compression4,18-32, but the studies have all been performed with some bed rest after the procedure (two hours or more).
Based on our data, patients can be mobilised immediately. PCI procedure was associated with a higher rate of complications, as reported earlier17,33,34), but, importantly, there was no association with immediate ambulation. Absence of diabetes was a predictor of complications other than small bleedings, something not previously reported; it could be a random finding.
Our findings are important for the post-interventional management of patients and imply that most patients can be mobilised immediately after the use of a VCD –if not contraindicated for other reasons. The prerequisite for immediate mobilisation is a successful deployment of the VCD after an uncomplicated procedure in a stable patient who is able to stand up and walk. In the randomised trial 11% of the screened patients did not meet these criteria. In the implementation study 87% of the non-emergent procedures with successful deployment of the VCD were such patients.
Therefore, non-acute patients undergoing coronary angiography and/or PCI can often be managed in an outpatient clinic as ambulatory patients if observation is not indicated for other reasons.
Strengths and limitations
The strength of our study is the combination of two study designs with a randomised trial to establish overall safety and the prospective validation registry showing the same overall results when the method is applied to an unselected population in an intention-to-treat design.
However, it can be debated whether the two study groups were completely comparable as the studies were undertaken in two different countries and there was also a considerable time span between the studies. Moreover, the sample size could have been too small to detect statistically significant differences between the randomised groups. Nevertheless, to date, the present study is the largest investigating complications after VCD implantation and indicating similar clinically important complication rates whether using the immediate or delayed mobilisation approach.
Conclusion
In patients undergoing coronary angiography or PCI, the use of immediate mobilisation after Angio-Seal™ deployment is safe with no significant differences in complication rates. With routine use of a femoral VCD, approximately 87% of patients are suitable for immediate mobilisation, enabling rapid discharge and increasing efficiency by reducing time spending in a typical cardiac catheterisation laboratory.
Funding
This work was supported by the University Hospital Basel and Gentofte University Hospital. There was a small unrestricted grant from St. Jude Medical Inc., St. Paul, MN, USA.
Conflict of interest statement
The authors have no conflict of interest to declare.

