Abstract
Aims: Vascular closure devices (VCD) have been introduced into clinical practice with the aim of increasing the procedural efficiency and clinical safety of coronary angiography. However, clinical studies comparing VCD and manual compression have yielded mixed results, and large randomised clinical trials comparing the two strategies are missing. Moreover, comparative efficacy studies between different VCD in routine clinical use are lacking.
Methods and results: The Instrumental Sealing of ARterial puncture site – CLOSURE device versus manual compression (ISAR-CLOSURE) trial is a prospective, randomised clinical trial designed to compare the outcomes associated with the use of VCD or manual compression to achieve femoral haemostasis. The test hypothesis is that femoral haemostasis after coronary angiography achieved using VCD is not inferior to manual compression in terms of access-site-related vascular complications. Patients undergoing coronary angiography via the common femoral artery will be randomised in a 1:1:1 fashion to receive FemoSeal VCD, EXOSEAL VCD or manual compression. The primary endpoint is the incidence of the composite of arterial access-related complications (haematoma ≥5 cm, pseudoaneurysm, arteriovenous fistula, access-site-related bleeding, acute ipsilateral leg ischaemia, the need for vascular surgical/interventional treatment or documented local infection) at 30 days after randomisation. According to power calculations based on non-inferiority hypothesis testing, enrolment of 4,500 patients is planned. The trial is registered at www.clinicaltrials.gov (study identifier: NCT01389375).
Conclusions: The safety of VCD as compared to manual compression in patients undergoing transfemoral coronary angiography remains an issue of clinical equipoise. The aim of the ISAR-CLOSURE trial is to assess whether femoral haemostasis achieved through the use of VCD is non-inferior to manual compression in terms of access-site-related vascular complications.
Introduction
Since their introduction into clinical practice, invasive coronary angiography and percutaneous coronary intervention have revolutionised the diagnosis and treatment of coronary artery disease (CAD)1,2. However, the techniques used to gain access to the vascular system as well as to achieve closure of the arteriotomy at the end of the catheterisation are critical elements of procedural success3. Specifically, as the human arterial circulatory system is a high-pressure system, failure to achieve prompt closure of the arterial puncture site may be associated with a spectrum of adverse events ranging from soft tissue haematoma and vessel wall pseudoaneurysm to potentially life-threatening retroperitoneal haemorrhage4. Such events may significantly impact on the overall risk to benefit ratio of coronary diagnostic and interventional procedures.
Manual compression remains the most frequently used modality for closure of arteriotomy after diagnostic catheterisation or percutaneous interventions3. However, the principal drawbacks are that the arteriotomy is not directly visualised and that compressive force is delivered at some remove from the actual puncture site. In addition, as the arteriotomy is initially occluded only by an unstable platelet clot, subsequent bed-rest of several hours duration is mandatory to prevent re-bleeding at the puncture site. Moreover, manual compression is often associated with considerable patient discomfort.
By interacting directly with the arteriotomy site, vascular closure devices (VCD) offer enhanced control of access-site haemostasis and potential for reduced access-site complications3,5. The two principal reasons for using a VCD are to enhance the efficacy of the percutaneous procedure and to improve the safety. Increased efficacy is usually defined as reduction in time to haemostasis, time to ambulation or discharge from hospital. Improved safety is usually defined as reduction in access-site bleeding and complications.
Over the last 20 years, several VCD systems have been developed and tested in clinical trials. However, partly due to negative experiences with certain devices, as well as lack of clinical trial data demonstrating improvement in patient outcomes and issues related to device cost, their adoption in routine practice has not been widespread. For example, a US registry reported that VCD use was limited to around 30% of percutaneous procedures performed in the country6. Indeed, results from early large-scale registries showed an increase in bleeding and a trend toward an increased need for vascular surgical repair associated with VCD use7. Concerns raised by such registries led the Food and Drug Administration to conduct reviews of patients’ outcomes after VCD using the ACC National Cardiovascular Data Registry. The results suggested that VCD and standard manual compression are associated with similar safety outcomes overall, with the exception of one VCD type that is no longer in clinical use8,9.
A number of meta-analyses have evaluated outcomes associated with VCD use. The analysis of Koreny et al showed that both VCD and manual compression were associated with similar rates of vascular complications10. A second meta-analysis performed by Nikolski et al showed similar vascular complication rates with VCD, independently of the type of procedure (diagnostic or interventional)11. Similar findings were observed in a more recent updated meta-analysis of 31 trials performed by Biancari et al12. However, all of these analyses are limited by the nature of the individual included trials which tended to be small, heterogeneous in design and performed in an era not representative of contemporary catheterisation procedures. Moreover, data were not captured on newer VCD which have entered routine clinical practice in recent years.
Overall, clinical studies comparing VCD and manual compression have tended to be of variable quality and have yielded mixed results13,14. Large-scale randomised clinical trials comparing contemporary VCD use and manual compression are lacking. Moreover, direct comparative efficacy studies between different VCD in routine clinical use are missing. Against this background we designed the Instrumental Sealing of ARterial puncture site – CLOSURE device versus manual compression (ISAR-CLOSURE) randomised trial.
Study design
The ISAR-CLOSURE study is a prospective, randomised clinical trial designed to compare patient outcomes after treatment with two different types of VCD, FemoSeal® (St. Jude Medical Systems, St. Paul, MN, USA), and EXOSEAL® (Cordis Corporation, Bridgewater, NJ, USA), compared with standard manual compression after diagnostic coronary angiography performed through the femoral access route. The hypothesis to be tested is that VCD are not inferior to manual compression in terms of access-site-related vascular complications. Demonstration of non-inferiority in terms of vascular complications might provide a rationale for the use of these devices on the basis of improvements in procedural efficiency, time to mobilisation and time to hospital discharge. We specifically chose to assess a patient population undergoing diagnostic catheterisation rather than coronary intervention. This is because periprocedural antithrombotic and antiplatelet therapy varies widely between different centres and operators and according to clinical presentations. This makes assessment of the specific contribution of closure devices difficult to interpret.
Study population
The study population will consist of 4,500 patients undergoing diagnostic coronary angiography via the common femoral artery. After performing a femoral angiography and verifying fulfilment of the inclusion criteria, the patients will be randomised in a 1:1:1 ratio to arteriotomy closure with FemoSeal, arteriotomy closure with EXOSEAL or standard manual compression. Further details of the inclusion and exclusion criteria of the study are provided in Table 1.
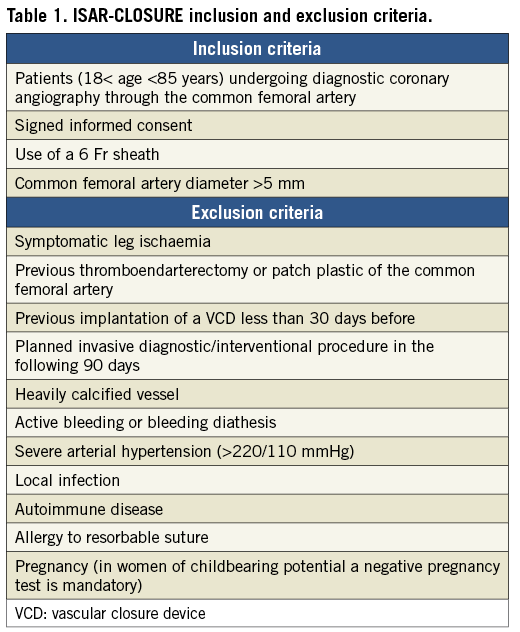
Details of vascular closure device systems
FemoSeal is an intravascular VCD, comprising an intravascular bioabsorbable polymer anchor plate and an outer disc, which are positioned respectively on the inner and outer surface of the artery and held together by a bioabsorbable filament, providing sealing of the arteriotomy (Figure 1A). The VCD is deployed over an exchange wire after removal of the standard vascular access sheath. The absorption of the plates and filaments is completed in approximately 90 days.
EXOSEAL is an extravascular VCD consisting of a bioabsorbable poly-glycolic acid plug, which is released on the external surface of the vessel. There is no residual anchoring component remaining inside the artery (Figure 1B). No sheath exchange is required. Two visual indicators on the shaft enable correct extravascular positioning of the plug. Light additional compression is required during the time taken for the plug to expand to fill the subcutaneous puncture tract. The absorption of the plug is completed in approximately 60-90 days.
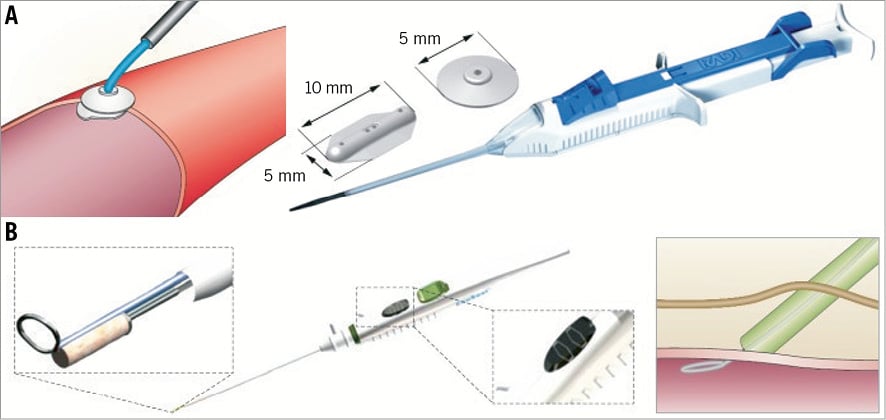
Figure 1. Study devices. A) FemoSeal® (St. Jude Medical, St. Paul, MN, USA). Comprises a bioabsorbable polymer anchor plate (10 mm×5 mm) and an outer disc (5 mm diameter). After procedural sheath removal the anchor seal is deployed within the artery whilst the outer locking disc is placed on the outer wall of the artery. The arteriotomy is sandwiched between the two components that are held together by a bioabsorbable multifilament. B) EXOSEAL® (Cordis Corporation, Bridgewater, NJ, USA). The device is advanced through the standard procedural sheath and the wire loop deployed inside the artery. A bioabsorbable plug (right panel) is released on the surface of the vessel (left upper panel) with two visual signals on the shaft to ensure extravascular deployment of the plug (middle panel, inset). Complete release is obtained through a button on the shaft. Modified from Byrne et al3 with permission.
Study procedures and randomisation
At the beginning of the procedure, the femoral artery will be accessed using a standard anterior wall puncture technique, and a 6 Fr access sheath will be placed in the common femoral artery using the modified Seldinger technique15. Angiographic or ultrasound guidance will not be routinely used to facilitate vessel puncture.
At the end of the procedure, patients meeting the eligibility criteria will be randomised in the order they qualify provided that signed informed consent has been obtained. The time of randomisation will be the termination of the diagnostic catheterisation procedure after the acquisition of angiography with injection of contrast through the arterial access sheath. Randomisation will be performed among the three treatment strategies with a randomisation sequence of 1:1:1 (1 FemoSeal: 1 EXOSEAL: 1 manual compression) (Figure 2). Randomisation will be stratified according to enrolling centre. Allocation to treatment will be performed by means of sealed opaque envelopes containing a computer-generated sequence. Patients will be considered enrolled in the study and eligible for the final intention-to-treat analysis at the time of randomisation.
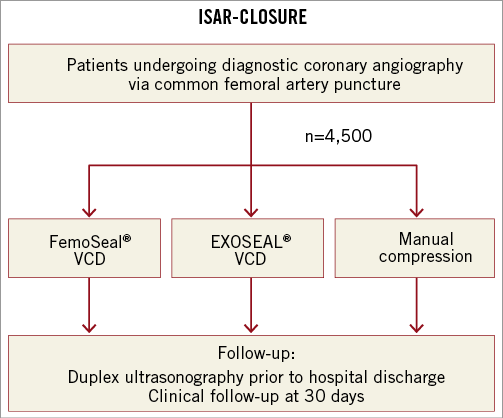
Figure 2. ISAR-CLOSURE study design. VCD: vascular closure device
In patients randomised to VCD therapy, the VCD will be deployed according to the standard device instructions for use under strictly sterile conditions. Enrolment of patients started after a run-in phase with these devices of at least six months after each of the operators received certification from the manufacturers of the respective closure devices. Thereafter, a pressure bandage will be applied for two hours. After removal of the pressure bandage and a careful clinical inspection of the access site by a doctor, the clinical findings will be documented and mobilisation will be allowed. In case an adequate haemostasis is not achieved through the use of VCD or if technical defects are detected, device failure will be noted and haemostasis will be achieved using manual compression. In patients randomised to manual compression the sheath will be removed immediately and groin compression will be performed by a physician until an adequate local haemostasis has been achieved. Subsequently, a pressure bandage will be applied over the puncture site for a duration of six hours. After removal of the pressure bandage and a careful clinical inspection of the access site, the clinical findings will be documented and mobilisation will be allowed.
The post-procedural clinical monitoring will include evaluation of pulse status, blood pressure, bleeding or haematoma at the access site. All patients will be scheduled to undergo a detailed colour-Doppler ultrasonographic evaluation of the common, superficial and deep femoral arteries prior to hospital discharge. A further clinical evaluation will be completed after a 30-day time interval. In case complications are detected, the patients will be invited for targeted clinical and ultrasonographic examination and/or source documentation will be obtained.
Primary and secondary endpoints
The primary endpoint of the study is the incidence of vascular complications at the vascular access site. Vascular complications are defined as the presence of any of: haematoma ≥5 cm, pseudoaneurysm, arteriovenous fistula, access-site-related bleeding (according to the REPLACE study definition16), acute ipsilateral leg ischaemia, the need for vascular surgical/interventional treatment or documented local infection. The secondary endpoints are time to haemostasis after sheath removal, failure of the vascular closure device to achieve adequate haemostasis and necessity of repeated manual compression. Details of endpoint definitions are provided in Table 2.
Ethics and informed consent
The study will be performed in accordance with the provisions of the Declaration of Helsinki17 as well as with the International Conference on Harmonization “Good Clinical Practices”. Before the commencement of the study, medical ethics committee approval for the protocol and the informed consent form was obtained. Written, informed consent from the patient is a prerequisite to participation in the study.
Statistical analysis
The study is designed to test the hypothesis that achievement of femoral arterial haemostasis using VCD is not inferior to manual compression in terms of incidence of vascular complications at the access site. The null hypothesis is that VCD is inferior to manual compression; the alternative hypothesis is that VCD is non-inferior to manual compression. The reported incidence of access-site-related bleeding and complications varies widely10. Based on the data reported in the literature and on our personal experience in a large number of patients assessed by dedicated surveillance protocols, the incidence of the primary endpoint was assumed to be 5% in the manual compression group. With an absolute non-inferiority margin of 2%, for a power of 80% and a one-sided a level of 0.025, we estimated that a total of 4,500 patients need to be enrolled in the study. Sample size calculation was performed with nQuery Advisor (Statistical Solutions, Cork, Ireland).
Categorical variables will be summarised using sums and percentage values, while continuous data will be summarised using mean value ±standard deviation or median (interquartile range). Demographic and angiographic data as well as procedural and ultrasonographic data will be compared using the Student’s t-test or Wilcoxon rank sum test (continuous data) or the c2 test (categorical data). Analyses will be performed according to the intention-to-treat principle. As analysis per protocol may be more conservative in trials with non-inferiority hypothesis testing, per protocol analysis will also be undertaken.
The primary analysis will compare manual compression and VCD regarding the primary and secondary endpoints. The secondary analysis will evaluate differences between the two vascular closure devices with regard to primary and secondary endpoints. A p-value <0.025 will be considered statistically significant.
Study limitations
The results of the current study relate to patients undergoing catheterisation via the femoral access approach. Although an increasing number of procedures are being performed via radial access, femoral access is still widely used and remains the dominant access in most centres worldwide. Moreover, the choice of sheath size in femoral access catheterisation is subject to some variability. Whether the results of the present study, enrolling patients undergoing diagnostic coronary catheterisation procedures through a 6 Fr access sheath, can be extended to procedures with smaller sheath sizes remains subject to future investigation. For reasons already discussed we specifically chose to assess a patient population undergoing diagnostic catheterisation rather than coronary intervention. However, data from the present study might serve as a basis for the design of further trials evaluating vascular closure devices in patients undergoing percutaneous interventional procedures.
Summary
Despite increased efficacy of VCD compared to manual compression, definitive evidence in terms of safety – defined as reduction in access-site-related bleeding and vascular complications – is still missing. The Instrumental Sealing of Arterial puncture site – CLOSURE device versus manual compression (ISAR-CLOSURE) trial is a randomised clinical trial which aims to enrol 4,500 patients to assess whether femoral haemostasis following coronary diagnostic angiography achieved through VCD is not inferior to manual compression in terms of access-site vascular complications.
Study organisation
Steering committee: Adnan Kastrati, MD (Chair); Maryam Linhardt, MD (Principal Investigator); Tareq Ibrahim, MD; Julinda Mehilli, MD.
Project management: Stefanie Schulz, MD; Julia Goedel, MD; Tobias Koppara, MD; Katharina Grupp, MD; Katharina Hoppe, MD.
Event adjudication committee: Olga Bruskina, MD (Chair); Andreas Stein, MD; Gjin Ndrepepa, MD.
Imaging core laboratory: Corinna Böttiger, MD; Phillip Groha, MD; Sebastian Kufner, MD.
Funding
Sponsor: Klinik für Herz-und Kreislauferkrankungen, Deutsches Herzzentrum, Munich, Germany.
Conflict of interest statement
A. Kastrati reports receiving speaker’s fees from St. Jude Medical. The other authors have no conflicts of interest to declare.

